Gene-therapy trial will attempt to restore hearing in deaf mice

Sound waves produce the sensation of hearing by vibrating hair-like structures on the inner ear’s sensory hair cells. But how this mechanical motion gets converted into electrical signals that go to our brains has long been a mystery.
Scientists have believed some undiscovered protein is involved. Such proteins have been identified for taste, smell and sight, but the protein required for hearing has been elusive. In part, that’s because it’s hard to get enough cells from the inner ear to study – they’re embedded deep in the cochlea.
“People have been looking for more than 30 years,” says Jeffrey Holt of the department of otolaryngology at Boston Children’s Hospital. “Five or six possibilities have come up, but didn’t pan out.”
Last week, in the Journal of Clinical Investigation, team led by Holt and Andrew Griffith, of the National Institute on Deafness and Other Communication Disorders (NIDCD), demonstrated that two related proteins, TMC1 and TMC2, are essential for normal hearing – paving the way for a test of gene therapy to reverse a type of genetic deafness.
The two proteins make up gateways known as ion channels, which sit atop the hair-like structures (a.k.a. stereocilia) and let electrically charged molecules move in to the cell, generating an electrical signal that ultimately travels to the brain. When both the TMC1 and the TMC2 genes are mutated, sound waves can’t be converted to electrical signals – they literally fall on deaf ears.
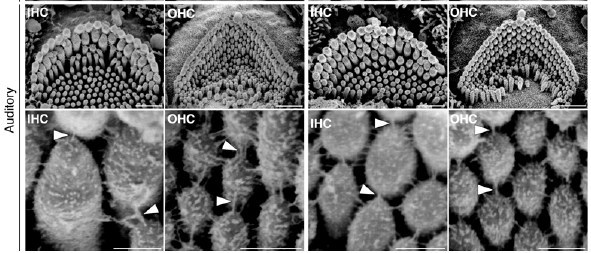
But in their experiments, Holt, Griffith and colleagues were able to restore these electrical signals in the sensory hair cells of deaf mice by introducing normal genes (carried in by an adenovirus). Using special techniques developed in Holt’s lab, they recorded electrical responses to noise when either TMC1 or TMC2 was added back – where before there had been none. “This is the first time anything like this has been done,” says Holt.
Thanks to previous work by Griffith and NIDCD-funded collaborators, TMC1 was already known to be mutated in a form of deafness in both mice and humans. TMC2 seems to have a redundant function and may compensate if TMC1 is defective, the new study showed.
But does restoring the electrical response restore actual hearing? Holt and collaborators at the Ecole Polytechnique Fédérale de Lausanne (EPFL) in Switzerland recently received a $600,000 grant for a gene-therapy trial in mice. The researchers will deliver TMC1 and/or TMC2 genes to the inner ear and measure whether electrical signals can be detected in the 8th cranial nerve and whether the animals respond to sound. EPFL will supply newer, safer gene-delivery vectors for testing that could potentially be developed for human trials.
Gene therapy has already been done in the eye, restoring vision in children and young adults with a genetic form of blindness, and another clinical trial is using gene therapy to deliver the drug Lucentis to patients with macular degeneration. But the ear is a more challenging organ with fewer sensory cells, says Holt.
“If we can restore hearing in a mouse and use the same vectors to put the gene in human tissue and restore function at the cellular level, it could pave the way for development of gene therapy strategies for treatment of human deafness,” Holt says. “That’s our long-range plan.”
Related Posts :
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

The dopamine reset: Restoring what’s missing in AADC deficiency
In March 2023, a young girl came to Boston Children’s Hospital unable to hold up her head — one striking symptom ...
-

Mark’s winning pass with cochlear implants
Mark Bradshaw wanted to break out of his parents’ protective shell — as many teens do when they start pushing for ...
-

A sickle cell first: Base editing, a new form of gene therapy, leaves Branden feeling ‘more than fine’
Though he doesn’t remember it, Branden Baptiste had his first sickle cell crisis at age 2. Through elementary school, he ...