Rapid saliva test detects COVID-19 variants, at home or point of care
COVID-19 tests are now widely available, including FDA-approved tests like BinaxNOW that people can do at home. But none of the home tests — or any hospital test — can distinguish between specific SARS-CoV-2 variants. Detecting and tracking variants, essential for public health efforts, requires complete nucleic acid sequencing of the virus. Currently, only specialized labs can do this, on a small sampling of positive COVID-19 swabs.
But in the future, a rapid, low-cost, CRISPR-based test platform called miSHERLOCK could provide reliable detection of specific SARS-CoV-2 variants in individuals — at home or in any health care setting.
Enhancing surveillance and clinical decision-making
Rose Lee, MD, an infectious disease physician at Boston Children’s Hospital, collaborated with the Wyss Institute for Biologically Inspired Engineering and the Massachusetts Institute of Technology to develop the platform. Having faced difficulties in testing patients early in the COVID-19 pandemic, she wanted a rapid, low-cost test technology that wouldn’t require complex processing in a central lab or depend on global supply chains for items like nasopharyngeal swabs.
And then the viral variants began emerging.
“It was a large group effort, since there was pressure to publish a variant-specific diagnostic rapidly,” says Lee, who is also a visiting fellow at the Wyss Institute. “Variant identification could provide more accurate and granular surveillance data for infection control and public health measures, and could be very helpful in making clinical care decisions, including choice of vaccine, the decision to use boosters, and use of monoclonal antibody therapies. Vaccines may differ in their protection against variants, and some monoclonal antibodies may be ineffective against certain variants because of changes in the spike protein that disrupt their binding.”
Rapid saliva-based variant testing
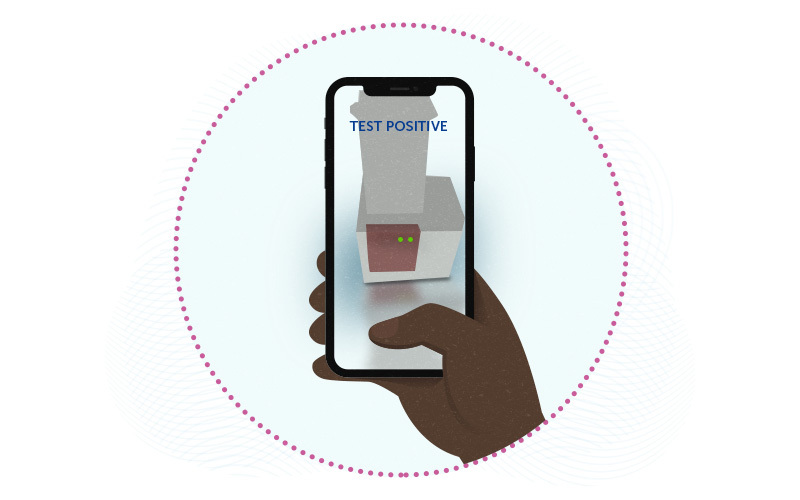
As described August 6th in Science Advances, miSHERLOCK (which stands for Minimally Instrumented Specific High Sensitivity Enzymatic Reporter Unlocking) consists of a small battery-powered device with two chambers. The user spits into one chamber, which purifies and concentrates the viral RNA. The second chamber then performs amplification and detection reactions. Within an hour, users get a visual fluorescent readout that they can interpret with an accompanying smartphone app. As new variants emerge, The test can be updated readily by plugging in new assays.
Lee, one of four co-first authors on the paper, played a key role in designing the device and the steps for processing users’ saliva, which includes chemical treatment to prevent false-positives from saliva enzymes. Colleagues in the Wyss Institute lab of Jim Collins, PhD, developed the CRISPR-based technology (described in detail in this press release) used to detect specific nucleic acid sequences characteristic of SARS-CoV-2 and its variants.
In tests on clinical samples, miSHERLOCK correctly identified COVID-19-positive patients 96 percent of the time and patients without disease 95 percent of the time, similar to the CDC’s gold-standard PCR assay. And it was able to distinguish between the alpha, beta, and gamma variants. An added assay to detect the delta variant is in the works.
Faster global tracking of COVID-19 variants
The researchers see huge potential for miSHERLOCK globally, especially in low-resource settings where the SARS-CoV-2 is circulating largely unchecked and more likely to spin off new variants. Users can 3D-print the test device from open-access online instructions, and mobile phone service is available virtually anywhere in the world. The team is also eager to work with manufacturers who could produce miSHERLOCK at scale for worldwide distribution.
“We pushed ourselves to create a truly decentralized, flexible, user-friendly diagnostic platform,” says Collins, senior investigator on the project. “By solving the sample prep problem, we’ve ensured that this device is virtually ready for consumers to use as-is, and we’re excited to work with industrial partners to make it commercially available.”
Helena de Puig, PhD; Devora Najjar; and Xiao Tan, MD, PhD of the Wyss Institute and MIT were co-first authors on the paper with Lee.
The study was funded by the Paul G. Allen Frontiers Group, the Wyss Institute, the National Institutes of Health, the Burroughs-Wellcome American Society of Tropical Medicine, and an American Gastroenterological Association Takeda Pharmaceutical Research Scholar Award.
Explore COVID-19 research at Boston Children’s Hospital
Related Posts :
-

A COVID-19 DNA nanoswitch: A new kind of test for a new kind of virus
When the COVID-19 pandemic shut down research laboratories across the country, several labs at Boston Children’s Hospital geared up, ...
-

A coming wave of diabetes? The link with COVID-19
Researchers are observing a new long-term health concern in patients hospitalized with COVID-19 — an increase in new-onset hyperglycemia lasting months ...
-

Boston Children’s post-COVID clinic cares for those with lasting symptoms
One teenager complains of chronic muscle pain. Another child feels too exhausted to go to school or play sports. Still ...
-

What drives severe lung inflammation in COVID-19?
A main feature of COVID-19 is lung inflammation and respiratory failure caused by an overexuberant immune response known as the ...