Avoiding a lifetime of injections: Can gene editing cure severe congenital neutropenia?

Fionn Mulrooney, a cheerful 11-month-old, in Plymouth, Massachusetts, has no idea he has a life-threatening genetic disease. Nor does he seem fazed by the daily subcutaneous injections his parents have learned how to give him. And little does he know that cells from his bone marrow are helping scientists develop an innovative gene-editing approach that could someday correct his disease, known as severe congenital neutropenia or SCN.
Weeks after he was born last year, Fionn spiked a fever that landed him in the local emergency room. He was transferred to a nearby hospital, but because of his racing heartbeat, he was later transported to the newborn intensive care unit (NICU) at Boston Children’s Hospital. At Boston Children’s, Fionn had a spinal tap and tests for multiple bacterial and viral infections.
“Nothing was testing positive, so they weren’t sure what was going on,” says his mother, Caitlin. “Everyone was waiting for his COVID-19 test to come back.”
That test was negative, too. But another very high fever followed two weeks later. Fionn’s parents rushed him back to Boston Children’s, where blood work revealed that he had virtually no neutrophils, white blood cells that normally fight infections. Without neutrophils, even normally harmless bacteria on the skin can become a serious threat. Fionn was evaluated in the Bone Marrow Failure and Myelodysplastic Syndrome Clinic, directed by Dr. Akiko Shimamura and part of the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center.
Specialized testing, including genetic testing, revealed the diagnosis of SCN, and Fionn began daily injections of granulocyte colony stimulating factor (G-CSF) to stimulate his bone marrow to make neutrophils.
“Before G-CSF, children with SCN used to succumb to severe, overwhelming bacterial and fungal infections,” says Shimamura.
Making neutrophils again
For now, Fionn is thriving and fever-free on G-CSF. But it’s an expensive treatment that can cause side effects, and may contribute to the increased risk of leukemia seen in children with SCN. There is a potential cure for SCN, bone marrow transplantation, if a well-matched marrow donor can be found. Fionn is lucky to have a perfect match in his brother Cillian, 3, but transplant has risks and complications, and many children lack a well-matched donor.
That’s where the gene editing approach may help. Dr. Daniel Bauer is experimenting with editing the DNA of stem cells from patients’ own bone marrow to enable the cells to make neutrophils. The edited stem cells would then be returned to the patient’s body, going back to the bone marrow.
Fionn’s parents agreed to donate cells from Fionn’s bone marrow biopsy to further Dr. Bauer’s work. “What’s exciting to me about this research is that potentially Fionn could have an option, short of stem cell transplant, that would allow his body to make neutrophils on its own,” says Caitlin. “I’m happy to donate the cells from his next biopsy as well.”
Bypassing the SCN mutation
Like many patients with SCN, Fionn has a mutation in a gene called ELANE. Hundreds of ELANE mutations are known, and all disrupt formation of neutrophils by altering the structure of a protein called elastase. The mutated elastase can’t fold into its proper configuration, causing the precursors of neutrophils to die rather than maturing into functional neutrophils.
Bauer’s gene editing approach, supported by a Blavatnik Therapeutics Challenge Award, bypasses this problem. Rather than adding a healthy ELANE gene, or using gene-editing tools to correct the ELANE mutation, Dr Bauer’s strategy introduces another mutation that knocks ELANE out completely. Dr. Bauer recently showed in the lab that this approach enables neutrophils to be made. If confirmed in human studies, it could potentially cure SCN caused by ELANE mutations.
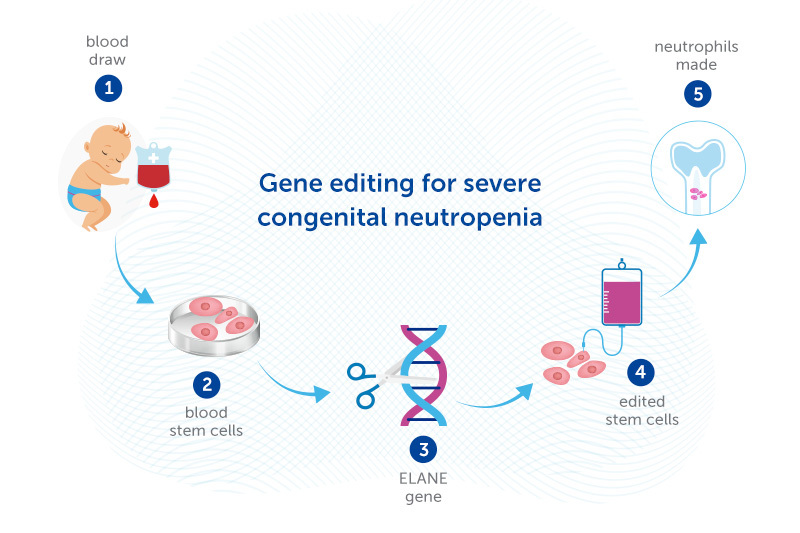
While the gene-edited cells can no longer make elastase, that turns out not to matter, since other proteins can take over its functions. The key is that the mutant, mis-folding elastase is no longer there.
“Traditional gene therapy to add a healthy copy of the gene wouldn’t work for SCN, because you would still have this mutant form that’s interfering with neutrophil production,” notes Dr. Bauer.
Another advantage of Dr. Bauer’s approach: knocking out ELANE avoids the need to target patients’ ELANE mutations individually. “We think our more universal approach could work for all different types of ELANE mutations,” Bauer says.
A future without neutropenia?
Drs. Bauer and Shimamura hope the approach can be tested in patients like Fionn within a few years. Fionn’s parents hope so too.
Meanwhile Fionn remains a healthy, happy baby, though of course he’s been largely sheltered at home during the COVID-19 pandemic. He plays with his brother Cillian, while his parents make sure the brothers aren’t sharing drinks, and that Cillian isn’t grabbing Fionn’s pacifier.

“Because he is at increased risk for bacterial infection, Fionn is generally supposed to avoid soil, pets, litter boxes, ponds, and other things with a high bacteria level, and we work to make sure that he and his environment remain clean,” says Caitlin. “At this age, he puts everything in his mouth, so I’m Clorox wiping all the time.”
In the fall, Cillian will start preschool and could bring home colds and ear infections, so the family will have another reason to be vigilant. But they remain optimistic.
“Dr. Shimamura said their goal for kids with neutropenia is not for them to be ‘bubble’ kids, but for them to live their lives,” Caitlin says.
Learn more about the Bone Marrow Failure and Myelodysplastic Syndrome Clinic
Related Posts :
-

Promising advances in fetal therapy for vein of Galen malformation
In 2024, Megan Ingram* of California and her husband were preparing for the birth of their third child when a 34-week ...
-

A true hero’s journey: How a team approach helped Wolfie overcome pancreatitis
Wolfgang, affectionately known as “Wolfie,” is a bright and energetic 7-year-old with a quick wit and a love for making ...
-

The hidden burden of solitude: How social withdrawal influences the adolescent brain
Adolescence is a period of social reorientation: a shift from a world centered on parents and family to one shaped ...
-

A toast to BRD4: How acidity changes the immune response
It started with wine. Or more precisely, a conversation about it. "My colleagues and I were talking about how some ...