Avoiding the needle: Engineering blood vessels to secrete drugs
People who rely on protein-based drugs often have to endure IV hookups or frequent injections, sometimes several times a week. And protein drugs – like Factor VIII and Factor IX for patients with hemophilia, alpha interferon for hepatitis C, interferon beta for multiple sclerosis — are very expensive.
What if they could be made by people’s own bodies?
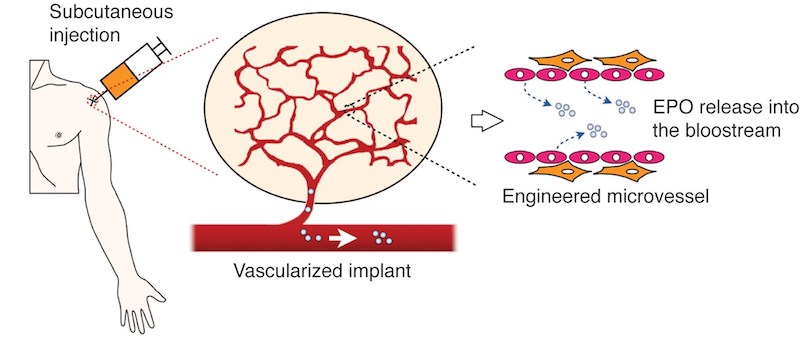
Combining tissue engineering with gene therapy, researchers at Children’s Hospital Boston showed that it’s possible to get blood vessels, made from genetically engineered cells, to secrete drugs on demand directly into the bloodstream. They proved the concept recently in the journal Blood, reversing anemia in mice with engineered vessels secreting erythropoietin (EPO).
This technology could potentially deliver other protein drugs, perhaps even insulin for diabetes. Right now, these drugs are produced by engineered cells in bioreactors, and come with a high price tag. “The paradigm shift here is, ‘why don’t we instruct your own cells to be the factory?’” says researcher Juan Melero-Martin, PhD, in the department of cardiac surgery at Children’s.
Melero-Martin and his colleagues built the drug-secreting vessels by isolating cells from human blood (namely, endothelial colony-forming cells), inserting some genetic code instructing them to make EPO, then adding mesenchymal stem cells. They suspended this mix in a gel and injected it into the mice, just under the skin.
The implanted cells spontaneously formed networks of blood vessels, lined with the engineered endothelial cells. Within a week, the vessels hooked up with the animals’ own vessels, releasing EPO into their blood. EPO circulated throughout the body and reversed anemia in the mice, significantly boosting their hematocrits as compared with controls.
“Endothelial cells are easily isolated from blood, are good at assembling themselves into blood vessels, and are ideal for releasing compounds into the bloodstream, since they line the blood vessels,” Melero-Martin says.
What’s more, the system has a built-in switch: The EPO-making gene didn’t turn on until mice received the drug doxycycline, which disabled a repressor protein that was linked to the gene. In fact, the researchers were able to turn EPO on and off at will – and make the animals’ hematocrit go up and down — just by adding doxycycline to their water on a weekly on/off schedule.
Since doxycycline is an antibiotic, which probably isn’t a great thing for people to take regularly, Melero-Martin and colleagues are looking at ways to either deliver doxycycline through the skin (to avoid exposing the whole body) or, better yet, build in on/off control through other means. One tantalizing possibility is to harness regulatory systems used naturally by the body – like those sensing blood oxygen levels and stimulating EPO production when oxygen levels dip.
Melero-Martin has built up considerable expertise in growing blood vessels; in 2008, he and Joyce Bischoff of Children’s Vascular Biology Program reported growing the first functional blood vessels in mice using cells from human donors. Blood vessels have some real advantages for drug delivery, and for gene-therapy applications whose goal is to produce a missing protein: They provide a way for genetically altered cells to be integrated in the body and, more importantly, stay in place.
The next frontier – allowing the technology to be extended to such applications as insulin for diabetes — is to get cells to store up and release therapeutics at a moment’s notice. Melero-Martin is also interested in using engineered blood vessels to kick-start tissue regeneration, building in genes for factors that attract stem cells or induce cells to differentiate.
“Blood vessels are one of the few tissues where we have good control over engraftment,” Melero-Martin says. “And we have the ability to put instructions inside.”
Related Posts :
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

The dopamine reset: Restoring what’s missing in AADC deficiency
In March 2023, a young girl came to Boston Children’s Hospital unable to hold up her head — one striking symptom ...
-

Modeling urinary tract disorders on a chip: Zohreh Izadifar
When a new tissue sample arrives from the Department of Urology, the Boston Children’s Hospital lab of Zohreh Izadifar, ...
-

A small act of kindness: Blood donations get Sadie off the sidelines after her aplastic anemia diagnosis
In March of 2024, Sadie’s life was interrupted. A busy high school senior with classes to attend, soccer matches to ...