Neurosurgeon’s pioneering technique helps thousands of children

Michael Scott, MD, was a 29-year-old neurosurgery resident at Massachusetts General Hospital in Boston when he saw a patient with a condition he hadn’t learned about in medical school: a narrowing of the blood vessels entering the brain. It was 1970, and though Japanese doctors had described the condition a few years earlier, it would take time for the worldwide neurosurgery community to become aware of the disease and its devastating effects on the brain. They called it moyamoya.
Scott would go on to develop a new surgical technique for treating moyamoya — and continue to refine the procedure as neurosurgeon-in-chief at Boston Children’s Hospital. Now 78 and approaching the end of his career, he’s taken the time to look back on his decades of experience with the disease.
In a remarkable paper in the Journal of Neurosurgery: Pediatrics, he and his colleagues track 59 of Scott’s patients over a period of 20 years or more, making it the longest longitudinal study of its kind. The paper looks not only at the patients’ medical outcomes but also at their schooling, employment, and personal lives.
Four doctors, three lawyers, and five babies

That’s fitting, because Scott takes a deep personal interest in his patients. He keeps a computer database of all the patients he’s treated over the last 30 years. He stays in touch with most of them regularly by email, and he revels in the details of their lives.
“We have four doctors in this group of kids who were treated, and three lawyers. We’ve got people with graduate degrees. There’s a guy in Alaska who’s a PhD working in environmental sciences,” Scott says. “Even now, I’m getting more follow-up information on these patients and hearing about how they’re doing. We just had another woman who had a baby. We’ve had five babies now, and the kids are all doing well so far. That’s wonderful.”
Six of the 59 patients in the study have died, but all for reasons unrelated to their surgeries. The others are thriving to a degree that would have been unimaginable before Scott’s pioneering technique.
“I think Mike would be very proud to say — though he’s probably too humble to say it — that the work done here at Boston Children’s has changed the treatment of this disease for years to come,” says Ed Smith, MD, who trained under Scott and now performs many of the hospital’s moyamoya operations. “I mean, he has changed the practice of medicine.”
Traffic jam in the brain
Much of the blood supply to the brain is delivered by the two carotid arteries, one on each side of the neck. If those arteries narrow after entering the skull, the brain responds by sprouting many smaller blood vessels to compensate for the diminished blood supply. To the Japanese doctors who first saw x-rays of the condition, those tiny vessels resembled a “puff of smoke,” or, in Japanese, moyamoya.
Smith, co-director of the Cerebrovascular Surgery and Intervention Center, likens moyamoya to a traffic jam on a holiday weekend in Boston. If the main highway through the city gets backed up, drivers turn off onto side roads in search of a way around the obstruction.
“Those little side roads get swollen and congested with traffic,” Smith says. “In a similar way, with moyamoya, when the main blood vessels get narrowed down, the tiny collateral vessels — the little side roads — become congested with traffic, and that congestion shows up on x-ray. That’s what the puff of smoke is. They’re side roads swollen with traffic, or new roads that are being built in response to the demand by the brain.”
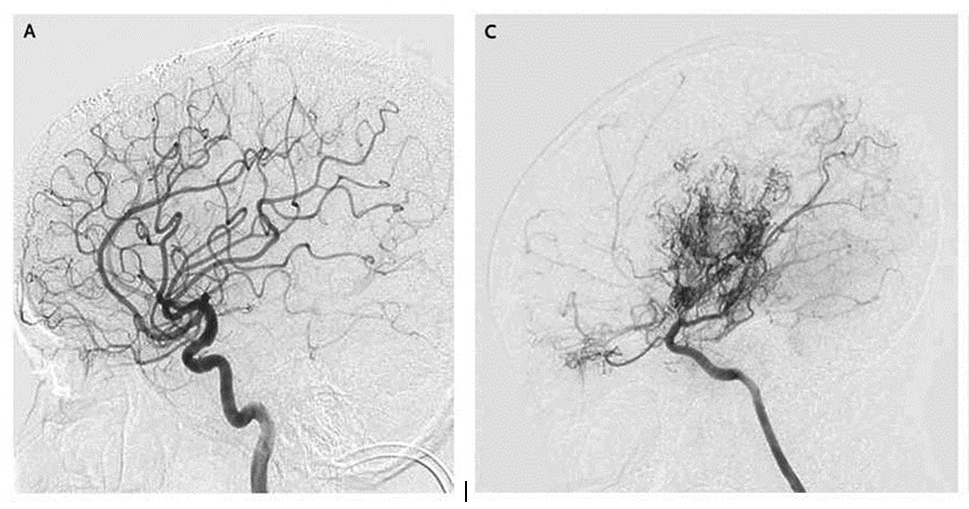
[IMAGES: COURTESY NEW ENGLAND JOURNAL OF MEDICINE]
Bleak prognosis
In the disease’s early stages, children with moyamoya often have no symptoms. But when there’s a sudden surge in demand for blood — during exercise, for example — the tiny blood vessels can’t handle the flow. In the worst case, the result is a stroke: the loss of blood to a certain part of the brain. “If a stroke happens in the speech center of the brain, a child can become mute or not understand language, and that can happen in a minute, literally,” Smith says. “And that can be a permanent effect for the rest of their life.”
Back in the 1970s and ‘80s, when Scott was first learning about the condition, he faced a lot of skepticism about moyamoya. “I had to do a lot of lectures before people really began understand, yeah, this does exist,” he recalls. “Everyone would say: ‘Stroke in children? That doesn’t happen.’ But it does.”
And stroke is not the only consequence. If left untreated, children with moyamoya face increased risks of sudden death, uncontrollable seizures, paralysis, cerebral hemorrhages, lowered IQ, and other cognitive problems. “The chronic loss of blood supply to the brain leads to the brain shrinking, because it can’t grow,” Smith says. “Just like if you have a plant and you don’t give it enough water, it’s never going to be as big and strong as one that gets regularly watered and fed.”
“I said, something’s wrong here”
Because the outlook for children was so bleak, Japanese doctors developed a surgical technique for treating moyamoya. They drilled a hole in the skull; cut a channel through the dura, the leathery covering of the brain; and rerouted a blood vessel from the scalp, laying it down in that channel in the hope it would sprout new branches providing more blood to the brain.
Scott, then a neurosurgeon at Tufts Medical Center, first performed the Japanese operation in 1983 on a 13-month-old girl who had already had three strokes. But the procedure didn’t work well for her or two other patients. “I said, something’s wrong here,” he recalls.
What was wrong, Scott realized, was that the Japanese surgeons hadn’t gone far enough. There was still one more layer between the blood vessel and the brain: a cellophane-like membrane called the arachnoid.
A surgical breakthrough
So in 1985, Scott made two modifications. First, he carefully opened up the arachnoid, bringing the donor blood vessel into direct contact with the pia — the surface of the brain itself. And second, he used tiny stitches to hold the blood vessel down, keeping it in contact with the brain.
“Those two additions made all the difference in the world,” he says. “We studied the children a year after surgery, and we were getting a plethora of new blood vessels going into the brain. It was a big success, and that’s what we’ve done ever since.”
“What Mike sort of intuitively figured out,” Smith says, “was that by very meticulously opening up that Saran Wrap on the brain, you’re doing two things: You’re removing a mechanical barrier to ingrowth. But more importantly, there are all these growth factors that the brain is squirting out, saying, ‘I’m hungry. Feed me!’ in response to chronic low blood supply. And by opening the arachnoid, you permit this fluid to get to the graft.”
Scott’s technique is called pial synangiosis — pial because it involves bringing the blood vessel into direct contact with the pia, and synangiosis because it capitalizes on the natural propensity of the blood-starved brain to attract new blood vessels (“angiosis”) from any source it can.
Building a team to care for patients

Since coming to Boston Children’s in 1988, Scott has performed the procedure on some 400 patients and worked with others at the hospital to improve the surgery and its outcomes. “The biggest role the hospital played was the development of a team to take care of the patients,” he says.
The team includes anesthesiologists, radiologists, and nurses, who not only care for the fragile young patients during and after surgery but also have written protocols now used by other hospitals performing the procedure.
Neurologists, too, play a key role by monitoring the children’s brain waves, allowing the surgeons to make adjustments throughout the operation. “We were one of the few, perhaps the only, group in the country that did that when this all started,” Scott says.
Lab research deepens understanding of disease
Meanwhile, Scott, Smith, and others have been working in the lab to understand why pial synangiosis works so well. They’ve identified some of the “Feed Me!” growth factors that are unleashed by the surgery. These proteins are normally active only before birth, while the embryo is building the brain and the blood vessels that nourish it.
“What we’ve found is that those same proteins can be pirated to repair a brain after birth, when there’s this physiologic stress of moyamoya,” Smith says. “The beauty of what we do here at Boston Children’s is that it’s this wonderful marriage of biology — this ancient program of how to build blood vessels — and what we do as surgeons. We’re simply creating an environment to allow nature to do what it does best.”
Epiphany bolstered by science
What Scott and Smith have learned in the lab — and the operating room — has also led to changes in how moyamoya is treated. For example, in the early days, when children presented with moyamoya on both sides of the brain, doctors would typically perform surgery only on the hemisphere where a stroke had already occurred. But in many cases the children would soon have a stroke in the other hemisphere. It’s often better, the surgeons learned, to operate on both sides at once.
Smith notes that the lessons learned at Boston Children’s have recently been incorporated into national guidelines that will inform and change practice at other centers around the country. It all began with Scott’s epiphany more than 30 years ago, he says. “And it’s been bolstered over the past decades through science and research that could only be done at Boston Children’s, because we have this unique combination of the patient population that gives us the people to study and the research labs that allow us to do the kind of bench-top research that other places can’t.”
A disease with many causes
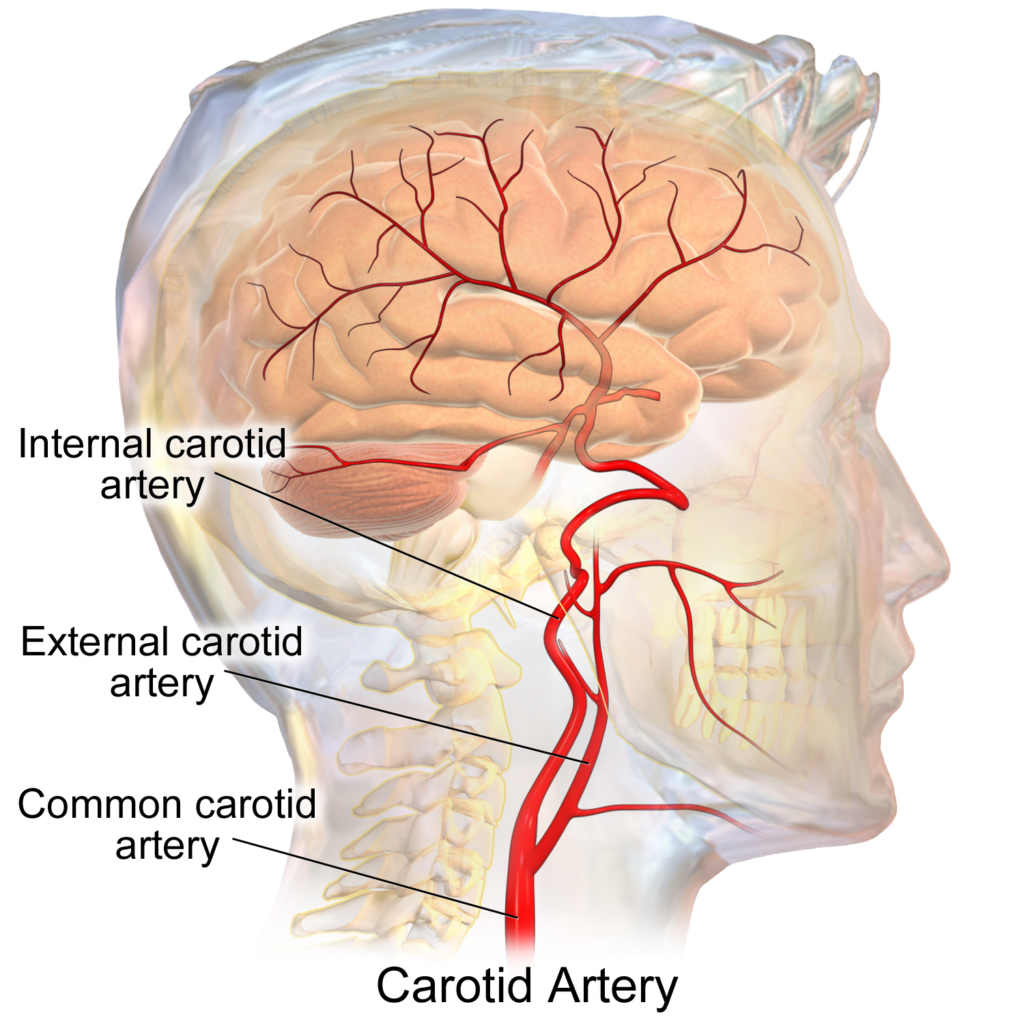
Any narrowing of the internal carotid artery, no matter what the cause, can lead to moymoya. [IMAGE: WIKIMEDIA COMMONS]
Moyamoya was once thought to affect only one in a million children born in the United States. But in the last decade, scientists have come to believe it’s at least ten times more common. This change is partly a result of greater awareness of the disease, partly a result of better imaging of the brain. But it’s also because it’s now known the disease may stem from a dozen different causes. “And we’re just now unearthing what those underlying causes are,” Smith says.
Most of the early cases were the result of a specific genetic mutation that is more common among Asian populations — that’s why the disease was discovered in Japan. But scientists now know certain conditions, including sickle cell disease and Down syndrome, can lead to the same narrowing of the blood vessels entering the brain. What all these causes seem to have in common is a mutation that triggers the smooth muscle cells in the walls of the carotid arteries to grow too fast. “It’s like putting too many bricks inside a tunnel,” Smith says. “It narrows the pipe down.”
Experience leads to better outcomes
Smith now treats 40 to 50 children a year for moyamoya — about three times the number of cases seen at Boston Children’s a decade ago. And the increase in volume, along with the research and clinical findings, have led to marked improvements in outcomes. In a study published in 2016, Smith and his co-authors showed that children treated at Boston Children’s and other “high volume” centers had far fewer complications during surgery, had shorter hospital stays, and were more likely to go home healthy than those treated at hospitals with fewer cases.
The long-term results are equally impressive. While at least two-thirds of untreated moyamoya patients typically suffer strokes within five years after diagnosis, all but one of Scott’s patients remained stroke-free and able to live productive lives. Of the surviving patients, 93 percent graduated from high school and 50 percent from college, 82 percent are living independently in adulthood, and 77 percent are working.
“What Mike’s paper shows,” Smith says, “is that, barring any complications, nature knows what to do. Once you treat them, this surgery will protect and preserve these children’s brains for the rest of their lives.”
Enduring legacy
Scott is pleased to have had the time to “write up his series” — to look back on the patients he’s treated for moyamoya and take stock of how they’ve done. He says one of his father’s greatest regrets was that illness prevented him from completing his own retrospective patient series at the end of his own career as a neurosurgeon.
“He always told me that it doesn’t matter how many papers you write or how many books you publish,” Scott says. “That’s stuff’s all going to be outdated in five or ten years. He said the most important thing is the patients you’ve taken care of and the people you’ve trained.
“I’ve trained people to do this procedure who are out now all over the country. They’re running their own programs in children’s hospitals. So I know that kids with this condition in good pediatric hospitals are getting good care.
I think Mike would be very proud to say — though he’s probably too humble to say it — that the work done here at Boston Children’s has changed the treatment of this disease for years to come. I mean, he has changed the practice of medicine.
Smith estimates that a thousand children have been treated for moyamoya at Boston Children’s alone since Scott developed pial synangiosis. And the spread of the technique to hospitals around the world means that thousands more children can now look forward to normal, healthy lives they would otherwise not have had.
“It’s been a great pleasure to be part of something like this,” Scott says. “And the follow-up I get from the patients is tremendously enjoyable. I come home to my wife and tell her I heard from a patient I operated on 25 years ago and they’re doing fine. It’s not science, but that stuff is pretty nice. I mean, not many people get that in their lives.”
The first co-authors on the paper were Coleman P. Riordan, BS, Armide Storey, BS, and David J. Cote, BS, all research assistants in the Department of Neurosurgery. Scott and Smith were senior co-authors. The work was supported by The Lucas Warner Fund, The Fellows Family Fund, The Oxley Research Fund, The Chae Family Fund, and The Kids at Heart Fund.
Related Posts :
-

Unveiling the hidden impact of moyamoya disease: Brain injury without symptoms
Moyamoya disease — a rare, progressive condition that narrows the brain’s blood vessels — leads to an increased risk of stroke ...
-

What orthopedic trauma surgeons wish more parents knew about lawnmower injuries
Summer is full of delights: lemonade, ice cream, and fresh-cut grass to name a few. Unfortunately, the warmer months can ...
-

Could the falcine sinus hold the key to vein of Galen outcomes?
A Boston Children’s Hospital study uncovers how fetal magnetic resonance imaging (MRI) could be a game-changer in predicting outcomes ...
-

A better treatment for endometriosis could lie in migraine medications
Endometriosis is a common, mysterious, often painful condition in which tissue similar to the uterine lining grows outside the uterus, ...