The surprisingly specific genetics of joint disease
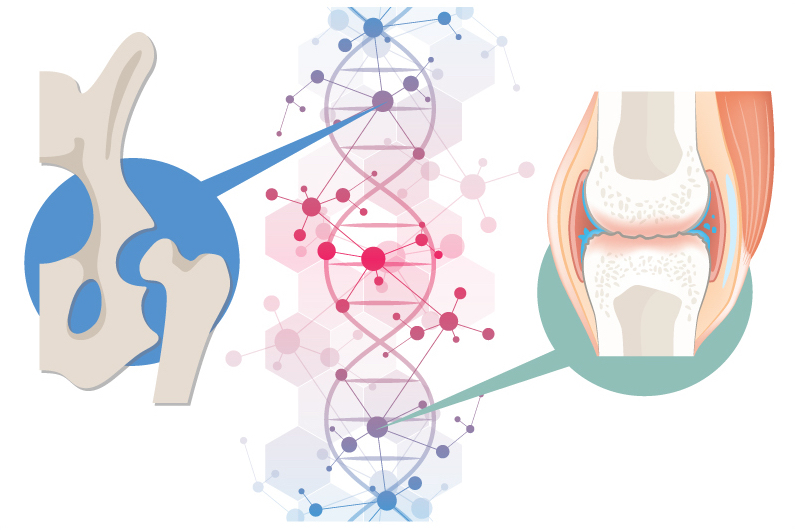
A new study provides unexpected insights into the biology of two common, heritable orthopedic conditions: developmental dysplasia of the hip (DDH) and knee osteoarthritis (OA). The findings, published July 6 in Nature Communications, show how a gene can have different effects in different parts of the body.
They also raise the possibility of preventive measures for people at genetic risk for DDH and knee OA, and perhaps other kinds of joint disease. They set the stage for more research on treatments targeting these genetic regulators or the biological pathways they affect.
From the lab to the clinical orthopedics clinic
Ata Kiapour, PhD, MMSc, director of the Musculoskeletal Informatics Group in the Orthopedic Center at Boston Children’s Hospital, saw an intriguing research opportunity when he attended a talk by evolutionary geneticist Terence Capellini, PhD, on joint disease associated with the gene GDF5. He realized he might be able to correlate Capellini’s laboratory findings in mice with clinical observations in patients with joint diseases.
Key takeaways
- Two genetic enhancers, each acting on the gene GDF5, are linked to developmental hip dysplasia and knee osteoarthritis, respectively.
- The findings raise the possibility of targeted preventive measures and perhaps therapeutics.
Kiapour and Capellini, from Harvard University’s Faculty of Arts and Sciences, had the same question. Why are certain joints more prone to disease and injury?
Thus began a joint effort that also involved the Broad Institute, the Harvard School of Dental Medicine, Harvard Medical School, and Dalian Medical University in China.
One gene, many effects
GDF5 plays a key role in joint formation. Genome-wide association studies (GWAS) in large populations have linked it with more than 20 traits and diseases affecting different parts of the skeleton. The strongest links are with DDH and knee OA.
But why these conditions? Both DDH and knee OA arise in part from altered biology of chondrocytes (cartilage cells), but little else was known. To delve deeper, Kiapour, Capellini, and their colleagues combined multiple techniques. These included genomic sequencing of human skeletal tissue, genetic studies in a mouse model, analysis of genetic data from patients with OA and DDH, and skeletal imaging data from both patients and mice with these conditions.
Suspecting that non-coding, regulatory parts of the genome may help direct GDF5’s action, the team also used a special assay called ATAC-seq to study chondrocytes from developing human hips and knees. They also studied chondrocytes from the corresponding locations in mouse embryos, looking for regulatory sequences shared in common.
Finally, they crunched the chondrocyte sequencing findings against GWAS data from patients with and without DDH and OA. This yielded two hits: variants in enhancers of GDF5, one active in the growth plate and hip joint, the other specific to the knee.
“Think of the gene as a light bulb with multiple different switches controlling where it is activated,” says Kiapour. “We showed that if you turn off the ‘knee switch,’ you get subtle morphological differences leading to knee OA. If you turn off the ‘hip switch,’ you get hip morphology seen in patients with hip dysplasia. You may have an intact gene, but enhancers may not switch it on where it’s needed.”
Can we intervene in hip dysplasia or knee osteoarthritis?
Closing the loop, Kiapour’s clinical imaging studies allowed the team to correlate enhancer mutations with anatomic findings in patients with DDH and knee OA. In the lab, testing different GDF5 expression levels in four engineered mouse lines produced parallel findings.
Together, the investigations provide insights into how variants in genetic enhancer regions cause early differences in joint shape as knee and hip joints are forming.
Think of the gene as a light bulb with multiple different switches. If you turn off the ‘knee switch,’ you get subtle morphological differences leading to knee OA. If you turn off the ‘hip switch,’ you get hip morphology seen in patients with hip dysplasia.”
So what does it all mean? Most immediately, Kiapour believes that knowing that a person has one of these enhancer mutations could spur preventive measures. In DDH, for example, more subtle forms may go unrecognized until they start causing problems in adolescence or adulthood. But if a patient has a genetic risk factor, lifestyle changes or other conservative strategies could potentially mitigate the risk, if started early enough.
“If we find through further studies that certain types of mechanical loading can influence joint shape when the skeleton is not yet fully mature, we can potentially develop activity modifications to prevent or reverse the effects of the genetic variants,” Kiapour says.
New therapeutics for joint disease?
Further down the road, if studies correlate genetic differences with responses to different drugs, patients could receive more personalized therapeutics. In addition, while DDH and OA have developmental origins, it’s possible that targeted treatments could modify GDF5 enhancers to shore up malformed joints and minimize arthritis later on. That will require more studies of GDF5’s role in joint maintenance.
“If further studies show that enhancers are active in joint maintenance, we may be able to develop a drug to control their expression and boost the regenerative capacity of cartilage and other joint tissues,” says Kiapour.
Learn more about orthopedic research at Boston Children’s Hospital
Related Posts :
-

Adam takes a pause from his active life for non-ossifying fibroma
Adam was 11 in early 2024 when he and his bike slid under a downed tree. Such events aren’t unusual for ...
-

New research paves the way to a better understanding of telomeres
Much the way the caps on the ends of a shoelace prevent it from fraying, telomeres — regions of repetitive DNA ...
-

New research sheds light on the genetic roots of amblyopia
For decades, amblyopia has been considered a disorder primarily caused by abnormal visual experiences early in life. But new research ...
-

Ask a sports medicine specialist: Why are ACL tears so common among female athletes?
When an athlete is sprinting after an opponent who suddenly stops or changes direction, their anterior cruciate ligaments (ACLs) make ...