Keeping up with HIV mutations: Building a nimble vaccine test system
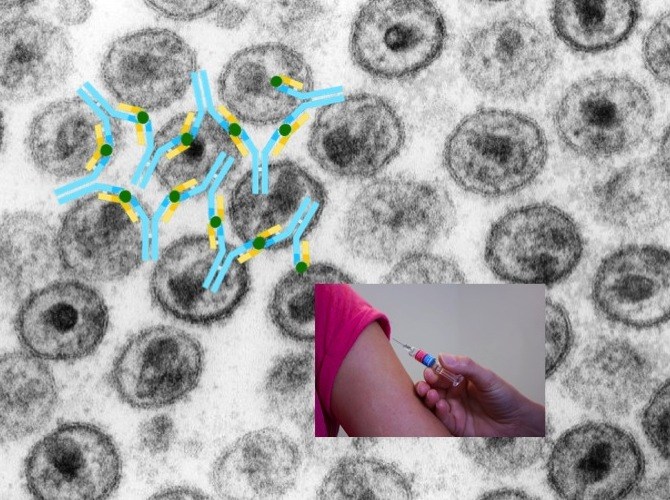
An AIDS vaccine able to fight any HIV strain has thus far eluded science. HIV frequently mutates its coat protein, dodging vaccine makers’ efforts to elicit sufficiently broadly neutralizing antibodies.
Yet sometimes HIV-infected people can produce such antibodies on their own. This usually requires years of exposure to the virus, allowing the immune system to modify its antibodies over time to keep up with HIV mutations. But the goal is generally achieved too late in the game to prevent them from being infected.
“Only a small fraction of patients are able to develop broadly neutralizing antibodies, and by the time they do, the virus has already integrated into the genomes of their T-cells,” says Ming Tian, PhD, of Boston Children’s Hospital’s Program in Cellular and Molecular Medicine (PCMM).
Tian is part of a group led by PCCM director Frederick Alt, PhD, that developed a technology to greatly speed up HIV development. Described today in Cell, the group’s method generates mouse models with built-in human immune systems. The model recapitulates what the human immune system does, only much more rapidly, enabling researchers to continuously test and tweak potential HIV vaccines.
A souped-up human immune system
People exposed to HIV (or any pathogen) first make precursor antibodies, which then mature via mutation and natural selection, an evolution-like process that makes them more protective over time.
“This is a long-term process, involving many intermediate antibodies, making it very challenging to design HIV vaccines to protect uninfected individuals,” says Alt, co-senior author on the paper with John Mascola, MD, director of the NIAID’s Vaccine Research Center (VRC). “To facilitate this effort, we wanted to design a new type of humanized mouse model that would be more physiological and allow us to very quickly test new vaccination strategies.”
How do broadly neutralizing antibodies to HIV naturally arise? The team began with the basic components of the known human antibody response to HIV.
Building a diverse immune repertoire
Our immune system’s B lymphocytes assemble antibody genes from building blocks known as V, D and J segments. Through various V-D-J combinations, our B lymphocytes are able to produce enormous numbers of different antibodies, enough to recognize almost any invader. After a B cell recognizes a pathogen, it further mutates the V-D-J sequence, often in successive steps, enabling its progeny B cells to produce even stronger antibodies.
Prior work had elucidated the structure of broadly neutralizing antibodies (bnABs) against HIV and deduced the V-D-J combinations that constituted their precursors. The team inserted the corresponding DNA into mouse embryonic stem cells.
The researchers then used the modified embryonic stem cells to rapidly generate mice whose B cells were primed to assemble a highly diverse set of HIV antibody precursors — combining the human precursor broadly neutralizing antibody “V” segment with various D or J segments.
An iterative process
The mice began by making immature “ancestor” antibodies. The VRC group, with collaborators at the Duke Human Vaccine Institute, the Scripps Research Institute, the Fred Hutchinson Cancer Research Center and other institutes, then sequentially exposed the Alt Lab’s test mice to a series of specially designed HIV antigens.
Through this sequential exposure, the animals’ B cells “learned” to produce ever more diverse and effective humanized antibodies that eventually were able to neutralize some HIV viral strains.
“Rather than go through generations of mouse breeding to make models, our approach allows us to quickly delete and replace genomic elements to create changes in B cells,” explains Alt. “Thus, we can rapidly re-program this mouse model with the intermediate antibody genes selected from the first successful immunizations, and expose them to new antigens. Over time, we hope this process will lead to the generation of broadly neutralizing HIV antibodies.”
As the engineered antigens engaged the system, upping the ante each time, the researchers could observe the antibodies acquiring mutations.
“You move the B cells in a direction and find out what works and the potential hangups,” says Alt. “You then work to figure out how to next adapt the mouse model and the immunogens to eventually get to a broadly neutralizing antibody stage.”
Speeding up AIDS vaccine development
There’s still a long way to go, but Alt believes the technology could hasten the search for a truly effective HIV vaccine, as well as vaccines against other viruses. It may also allow researchers to generate highly specific therapeutic antibodies. “We’re hoping it will be broadly useful,” Alt says.
Ming Tian, Cheng Cheng, Xuejun Chen and Hongying Duan, of the NIAID’s Vaccine Research Center (VRC), and Hwei-Ling Cheng of the Alt Lab were co-first authors on the paper.
The study was supported by the National Institutes of Health and the National Institute of Allergy and Infectious Diseases (R01AI077595, AI020047, P01 AI094419 U19 AI109632); the NIH Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) (AI100645, 5UM1AI100645, 1UM1 AI100663); the International AIDS Vaccine Initiative Neutralizing Antibody Consortium and Center; CAVD funding for the IAVI NAC Center; the Ragon Institute of MGH, MIT and Harvard; the VRC; and the Howard Hughes Medical Institute.
Related Posts :
-

Study highlights the severity of acute necrotizing encephalopathy in kids with the flu
For most children, influenza (flu) usually means unpleasant symptoms like a fever, sore throat, and achy muscles. But for a ...
-

A surprising link between Crohn’s disease and the Epstein-Barr virus
Crohn’s disease, a debilitating inflammatory bowel disease, has many known contributing factors, including bacterial changes in the microbiome that ...
-

Could peripheral neuropathy be stopped before it starts?
An increase in high-fat, high-fructose foods in people’s diets has contributed to a dramatic increase in type 2 diabetes. This, ...
-

Could we cure or prevent food allergy by targeting an intestinal protein?
When is food simply nourishing and enjoyable, and when does it provoke an allergic reaction? The answer appears to lie ...