Not all brain tumors are made the same, and that’s important

When you look at an apple, no matter what variety, on the surface you can be pretty sure it’s actually an apple. From there, you can make lots of assumptions about it, like how it will taste when you bite into it and what will happen if you plant the seeds in your yard.
With cancer, we can’t make those kinds of assumptions. While two tumors from the same location in two patients may look the same, doctors and researchers have come to recognize that their behavior and the mutations driving them can be radically different, as can their response to therapy.
With that recognition, physician/scientists like Scott Pomeroy, MD, PhD, the neurologist-in-chief at Boston Children’s Hospital, are taking a deeper look at the tumors they commonly see and asking whether what on the surface looks like one kind of tumor might actually be something completely different. Pomeroy in particular has applied this view to one of the biggest questions in pediatric cancer: Why do medulloblastomas, the most common malignant childhood brain tumor, behave so differently from child to child?
“When you look at these tumors under a microscope, they appear quite similar, but have marked differences in clinical outcome,” says Pomeroy, who has studied medulloblastomas for nearly 20 years. “We haven’t understood the biology driving prognosis, and how to adjust our treatment approach accordingly.”
Medulloblastomas: Divide and conquer
In 2010, Pomeroy and his collaborators at Dana-Farber/Children’s Hospital Cancer Center (DF/CHCC) got the first genomic inkling of how to “divide and conquer” medulloblastomas. In what was then the largest such study of medulloblastomas, Pomeroy’s team found that they could group tumors into six subtypes—essentially six different diseases—each with a unique molecular “fingerprint” (combinations of patterns of genetic activity (gene expression) and chromosome changes) and a distinct prognosis ranging from 97 percent survival down to a frightening low of 20 percent. The study was the first step to taking a personalized medicine approach to treating these tumors.
There’s since been consensus among brain tumor oncologists and neurologists that the six subtypes should be whittled down to four. But this was only a starting point. What mutations cause the molecular fingerprints of each subtype? The only way to know that would be to perform deep sequencing of the full complement of protein-coding genes (the “exome”) of a series of medulloblastoma tumors.
Which is what Pomeroy and his collaborators just did. In a paper just released in Nature, they reveal that by sequencing 92 tumors from patients with medulloblastoma, they could pin down the precise mutations driving the subtypes.
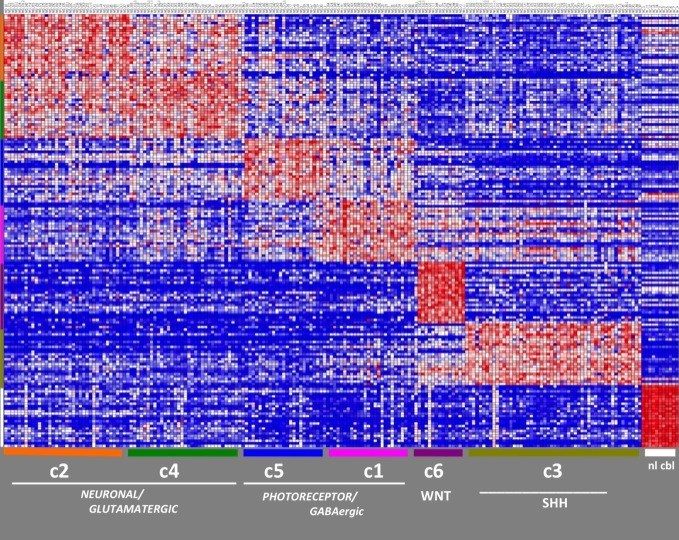
Apart from providing a set of discrete targets for biomarker and drug development, the data have also helped researchers understand a few common themes about what makes a medulloblastoma a medulloblastoma. First off, the mutated genes fell into two broad categories: genes like Wnt and the whimsically named Sonic hedgehog (Shh) that play direct roles in molecular pathways controlling cell growth, and genes like DDX3X and GPS2 that play more of a coaching role, modulating the activity of other genes.
But those categories harbored a surprising mix of actual mutations. The vast majority of the mutated genes they found were mutated only once in their entire tumor collection; in fact, only 12 genes were mutated in more than one tumor.
Taken as a whole, the team’s research confirms the view of medulloblastoma as a family of tumors driven by disruptions in just a few common mechanisms. However, the results point out that the form those disruptions take—the actual mutations or genomic changes—can vary from tumor to tumor.
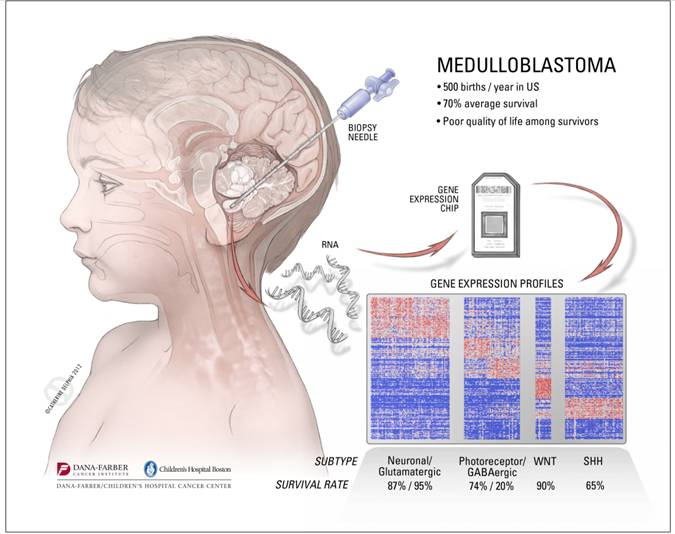
Pomeroy is confident that some of the study’s findings could be translated to patients relatively quickly. For instance, it should soon be possible to create a small panel of biomarkers that doctors could use to rapidly subtype individual medulloblastoma patients’ tumors and decide how aggressively to treat them. Also, drug companies are already working on Shh-blocking drugs that could be tested in Shh-fueled medulloblastomas within a couple of years.
“Not only do we now know how to stratify medulloblastomas genomically, we have a firm grasp of what gene mutations drive each molecular subtype,” he says. “For the first time, we’ll be able to classify and treat medulloblastoma based on molecular typing, providing the best therapy with the fewest long-term consequences.”
Related Posts :
-

New research sheds light on the genetic roots of amblyopia
For decades, amblyopia has been considered a disorder primarily caused by abnormal visual experiences early in life. But new research ...
-

Thanks to Carter and his family, people are talking about spastic paraplegia
Nine-year-old Carter may be the most devoted — and popular — sports fan in his Connecticut town. “He loves all sports,” ...
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...
-

Forecasting the future for childhood cancer survivors
Children are much more likely to survive cancer today than 50 years ago. Unfortunately, as adults, many of them develop cardiovascular ...