Diving deep: Understanding skeletal conditions with fish models

From fragile ice fish deep in the Antarctic Ocean to flying fish gliding above the Caribbean sea, fish have evolved a fascinating variety of skeletal traits. These traits not only help them adapt to their environments, they are also providing genetic insights into rare human skeletal disorders. Fish are not as genetically different from us as you might think, and collaborations between scientist Matthew Harris, PhD, and doctors and surgeons at Boston Children’s Hospital are starting to bridge the gap between the natural and clinical worlds.
Why fish?

Harris started out studying marine biology, then later moved into in developmental genetics, using evolutionary adaptations in fish to unlock the potential of the human genome. His research questions, like “Why do organisms maintain variations in their genome that are potentially harmful?” are very broad. But the answers could lead to practical applications in medicine, and perhaps new treatment approaches for children with skeletal anomalies.
In addition to studying fish in the wild, Harris’s lab develops zebrafish that accurately model human skeletal disorders to better understand the underlying mutations and biological mechanisms. Zebrafish make good models because they are small, easy to manipulate, inexpensive, breed quickly, and have a significant genetic overlap with humans — sharing about 80 percent of their genomes through a common ancestor.
“At one point, we were all fish,” says Harris.
Fish on the brain
John Meara, MD, DMD, with the Department of Plastic and Oral Surgery at Boston Children’s, provides Harris with leftover bone fragments from patients undergoing surgery for various craniofacial anomalies. One collaboration is around craniosynostosis, a condition in which the skull bones fuse together too early during a child’s development, causing problems with brain and skull growth.
Harris produces zebrafish with a variety of mutations, looking for cranial abnormalities similar to those in craniosynostosis. “He gets a lot of really cool looking fish that have different types of skull fusions,” says Meara.
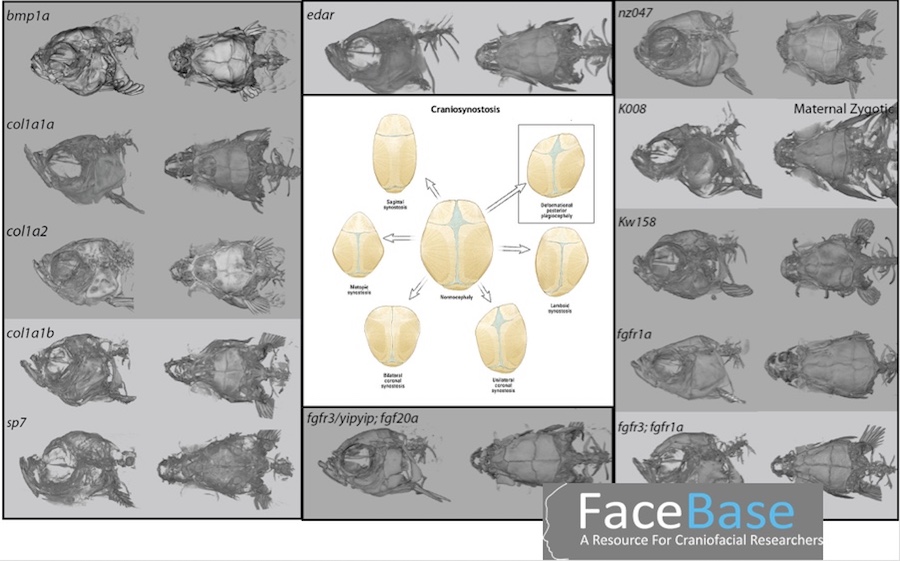
Harris then compares the zebrafish DNA with DNA from patient samples and pinpoints their shared mutations, which are useful for understanding the origins of craniosynostosis.
“To get transformational research, you need to link the clinicians who are seeing things in humans with the basic science researchers who have the ability to identify not only the mutations but the mechanisms causing a certain disease,” says Meara. “Together, that’s how you can make real progress.”
One fish, two fish…
Harris and his team have pursued answers all over the world, guided by the unique adaptations of the fish they study as well as the rare skeletal conditions of children seen at the hospital. In Antarctica, their research centers around deep water ice fish which have developed thin, brittle skeletons that allow them to float above the sea floor, despite the intense pressure of the water above. The fishes’ thin bones are similar to those of children with another rare disease treated at Boston Children’s: osteogenesis imperfecta, or brittle bone disease.
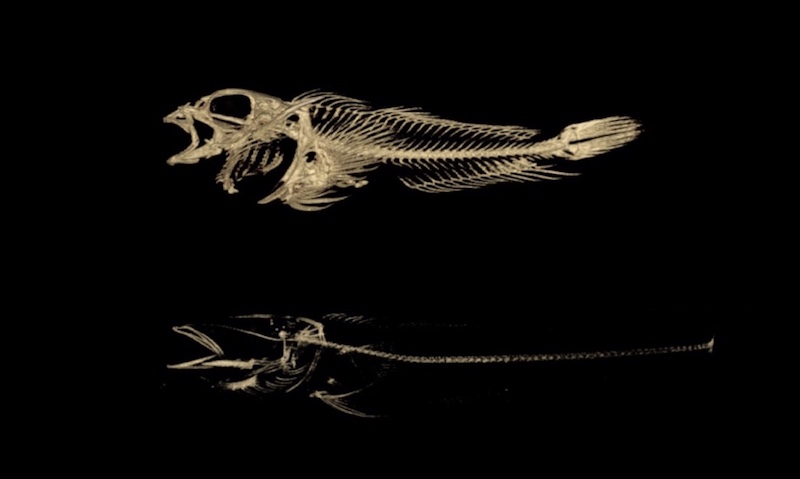
“The same change in a physiological process in one animal might be adaptive, and in the other animal might be pathological,” says Harris.
Harris and his team sequenced the genomes of 46 species of ice fish and their close relatives. By comparing specific genes across all of the species, the team was able to narrow down the exact “spelling changes” in DNA that led to the loss in bone density. The team then replicated these mutations in lab zebrafish and observed analogous changes in skeletal density that will serve as a model in future research.

Christina Jacobsen, MD, an endocrinologist and director of the Bone Program who does research in Boston Children’s department of Orthopedic Research, is currently collaborating with Harris to find genes that modify the severity of osteogenesis imperfecta.
“One child might have fractures very early in life and need medical therapy, while their sibling might have hardly any fractures in childhood, and do quite well,” she notes.
While Jacobsen has been using mouse models to look for modifying mutations that contribute to this variation, Harris is looking for similar modifications in zebrafish.
“We’re able to go at it from slightly different perspectives and give a little bit more power to our experiments by using two different models,” says Jacobsen.
Is it a bird? Is it a plane? No, it’s a fish!
Other research in the Harris lab is looking at flying fish, whose powerful fins allow them to glide above the water as far as 650 feet in one go, flying just out of reach of predators. Harris noticed that the long bones that support these kite-like fins are analogous to the elongated bones in patients with macrodactyly, a skeletal anomaly in which fingers or toes can grow up to three times their normal size.
In association with the Central Caribbean Marine Institute, lab members went down to the Caribbean to sequence the genomes of flying fish and related species, collectively known as needlefish. As with ice fish, the team identified specific mutations and recreated the same mutations in zebrafish. The zebrafish, too, grew longer fins.

Brian Labow, MD, a plastic surgeon in Boston Children’s Hand and Reconstructive Microsurgery Program, is working with Harris to compare the genetic mutations of flying fish with those found in bone samples from patients with macrodactyly. Until now, the genetics of macrodactyly have been difficult to study, because of its rarity and because the causative mutations don’t appear in every cell in the affected area. Harris can introduce the same mutations into developing zebrafish to investigate which combinations lead to this extreme localized overgrowth.
“It is very valuable to use whole animal models to study genes of interest and see whether or not you can generate your phenotype,” says Labow. “In macrodactyly, there has never been an animal model, ever, up until this collaboration with Matt.”

Hooked on longevity
Today, Harris is looking beyond the skeleton. Using rockfish as a case study, he is investigating human longevity. Rockfish, normally unassuming ocean bottom dwellers, can live up to 250 years, and Harris is intent on finding out why.
In all of his work, Harris maintains a passion for understanding human genetics and disease using the examples already provided by nature.
“When you get anywhere near this type of research, you find these underlying lessons of life,” he says, “and you really get hooked.”
Related Posts :
-

Hard and beautiful at the same time: Five lessons of raising a medically complex child
When they learned they were expecting a baby, Michelle and Stephen Strickland were delighted. The South Carolina couple looked forward ...
-

3D imaging could become standard practice in orthopedics. Here’s how.
It took a trained eye to see the abnormality on the patient’s X-ray. There, hidden behind the acetabulum was ...
-

A case for Kennedy — and for rapid genomic testing in every NICU
Kennedy was born in August 2025 after what her parents, John and Diana, describe as an uneventful pregnancy. Soon after delivery, ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...





