Bone marrow-on-a-chip provides new research directions for Shwachman-Diamond syndrome
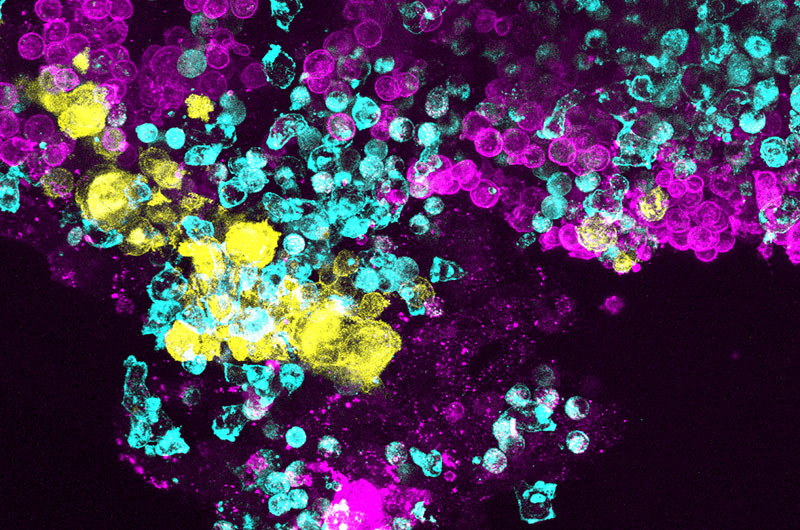
A new research tool that mimics the behavior of diseased bone marrow provides a new strategy for understanding the bone marrow disease, Shwachman-Diamond syndrome (SDS), and hopefully, developing new treatments. With SDS, bone marrow fails to produce blood cells normally, leading to bone marrow failure and an increased risk of leukemia.
In a research paper published January 27 in Nature Biomedical Engineering, researchers describe how damaged bone marrow from SDS patients performed in the new tool, known as the organ-on-a-chip (Organ Chip). The bone marrow Organ Chip was developed in the lab of Donald Ingber, MD, PhD, at the Wyss Institute for Biologically Inspired Engineering at Harvard University and a member of the Vascular Biology Program at Boston Children’s. The chip was evaluated with SDS bone marrow samples housed in the SDS Registry at Boston Children’s Hospital.
Lack of an effective animal study model

“We need to find better ways to treat SDS to either prevent leukemia or risk-stratify patients to figure out who would benefit from earlier intervention,” says co-author Akiko Shimamura, MD, PhD, director of the Bone Marrow Failure and Myelodysplastic Syndrome Program at the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. Shimamura also co-directs the SDS national registry maintained at Boston Children’s Hospital in collaboration with Dr. Kasiani Myers at Cincinnati Children’s.
However, progress has been slower than hoped because of the lack of a useful animal study model that could be used to mimic the behavior of SDS. Previous SDS animal studies produce very severe disease and animals die quickly, making it very difficult to research the medical complications that are typically found in SDS patients.
In the paper, Ingber, Shimamura, and colleagues describe how the bone marrow Organ Chip effectively provides a model system to develop new treatments for SDS.
“This model can be used for biological studies and for drug screening,” says Shimamura. “In my lab, we have identified some potential therapeutic targets for treating SDS that we hope to test with the new bone marrow chip.”
3D Bone marrow matrix
The Ingber group developed the marrow-on-a chip with two hollow, parallel channels divided by a semi-permeable membrane.
In the bone marrow study, the top channel was filled with blood-forming (hematopoietic) stem cells from the bone marrow of SDS patient and stromal cells, including bone marrow stem cells, derived from the bone marrow. Both are embedded in a matrix gel to mimic the three-dimensional nature of marrow tissue. The bottom chamber was lined with human endothelial cells to mimic the vasculature within bone marrow. A liquid medium that supports the growth of healthy bone marrow cells and their differentiation into many different blood cell types flows through the vascular channel. Healthy marrow was also applied to a set of organ chips to act as a healthy control group.

Chip mimicked SDS patient cells
In the SDS experiment, after two weeks the team observed that bone marrow on the SDS Organ Chip produced fewer blood cells, especially mature white blood cells, compared with chips from healthy donors.
“We found that the blood cell production from the SDS marrow was impaired in a manner that reflected what we see in patients,” says Shimamura.
The team also found a previously unknown abnormality in the SDS bone marrow cells on the Organ Chip. Maturing neutrophil white cells on the SDS chip did not switch on the CD13 gene as much as those on normal bone marrow. When researchers studied pathology reports of the SDS marrow from these same patients that are done as part of clinical care, they saw this abnormality was mirrored there. Over half of the SDS samples from patients had lower levels of CD13.
We hope to better understand the specific molecular events that are causing or driving the bone marrow failure in SDS. This system might provide deeper insights into what is happening molecularly in the cells of these patients that we did not know before. We now have a strong basis to pursue this further to figure out which agents should move forward into the clinic.
Akiko Shimamura
Shimamura’s next step is to see how the bone marrow Organ Chip responds to potential new therapies. “We are going to put patient marrow on the chip, and then flow candidate drugs through the vascular chamber to see if any of the drugs improve the marrow’s ability to produce blood cells,” she explains. “We can then remove the marrow from the chip and study the mechanism by which the drug is working and then develop better or more specific agents.”
In related SDS research, in late December 2019 Daniel Bauer, MD, PhD, an attending physician with the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center received an NIH grant to develop CRISPR-based gene editing as a potential definitive therapy for SDS patients. The marrow chip will be part of his experimental evaluation in collaboration with the SDS registry.
SDS registry collaboration

This latest paper details just one of the many ways in which SDS patients and families contribute to SDS research. At just over ten years old, the SDS registry is a close collaboration between SDS patients, their families, and researchers. The goal of the registry is to hasten the pace of SDS discoveries using donated bone marrow samples from SDS patients nationwide.
Funding for this project was provided by a grant from the National Institutes of Health.
The research was performed in collaboration with researchers at the Wyss Institute, Dana-Farber Cancer Institute, Massachusetts General Hospital, and AstraZeneca.
Learn more about one SDS family here.
Related Posts :
-

A case for Kennedy — and for rapid genomic testing in every NICU
Kennedy was born in August 2025 after what her parents, John and Diana, describe as an uneventful pregnancy. Soon after delivery, ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

The dopamine reset: Restoring what’s missing in AADC deficiency
In March 2023, a young girl came to Boston Children’s Hospital unable to hold up her head — one striking symptom ...
-

Thanks to Carter and his family, people are talking about spastic paraplegia
Nine-year-old Carter may be the most devoted — and popular — sports fan in his Connecticut town. “He loves all sports,” ...