Making history: Gene therapy for CCALD gives Conner a second chance

Like a lot of 6-year-olds, Conner Hess finds joy in simple acts: drawing pictures, cuddling his two cats, and playing video games with his father. When his fun times at home in New York are interrupted by trips to Boston Children’s Hospital, he knows it’s to help fix a “boo-boo” in his brain. What he doesn’t yet realize is that he’s making history during those appointments: On March 16, 2023, Conner became the first patient with childhood cerebral adrenoleukodystrophy (CCALD) in the world to receive the gene therapy SKYSONA outside of a clinical trial.

Rare condition, grim prognosis
Adrenoleukodystrophy is a rare genetic condition that destroys the protective myelin sheath around the brain’s nerve cells. Without the myelin sheath, the nerves can no longer relay information to and from the brain. CCALD, a common form of ALD, is the most devastating. It predominately affects boys, with symptoms developing between ages 4 and 10. Signs of CCALD can begin as poor school performance or behavioral problems but progress to vision difficulties, seizures, incontinence, and trouble moving and speaking. Untreated, CCALD typically leads to severe neurologic deterioration, disability, and death within a few years.
Like most families, Conner’s parents, Richelle and Adam, had never heard of CCALD until his middle brother was screened — and tested negative — for it as a newborn (see sidebar). When Conner was born three years later, he also underwent screening — but this time the results were much different.
“A neurologist called us and said she needed to see all three of our boys immediately,” remembers Richelle. Further testing confirmed that both Conner and his oldest brother, Jayden, had the genetic mutation for ALD. “I started Googling and reading all these horror stories,” she says. “I felt like we had been handed a death sentence.”
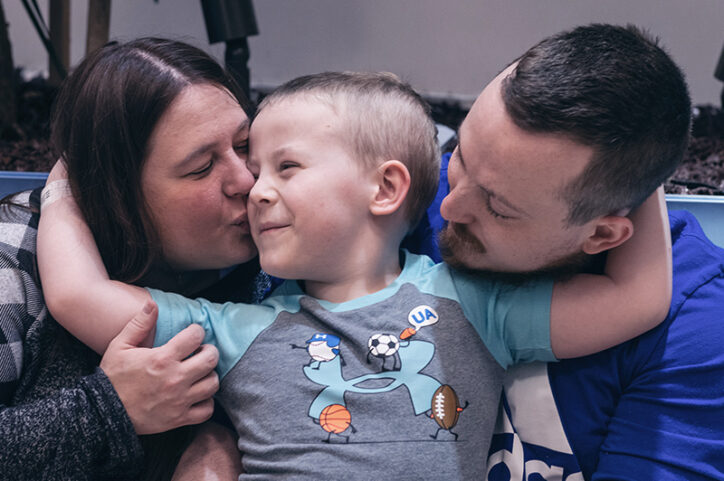
A race against time
Those test results triggered semiannual MRI scans for both Conner and Jayden, which would show whether the mutation was progressing to CCALD. Remarkably, Jayden, now 13, has never developed symptomatic CCALD. “We were hopeful that the same would be true for Conner,” says Richelle. “But it wasn’t.”
Last September, an MRI scan revealed the family’s “worst nightmare”: Conner had early signs of brain changes on scans suggesting progression of CCALD. Since his birth, Richelle had educated herself about CCALD and possible treatment options. Now, she assumed that Conner would undergo a stem cell transplant (also called a bone marrow transplant), which would provide him with healthy donor stem cells that produce a protein lacking in boys with CCALD. Although a stem cell transplant can help prevent further damage to the nervous system, it cannot reverse existing damage. Richelle and Adam knew the race was on to find a match.
Why newborn screening is key
Conner benefited from newborn screening for ALD. The test, which was first added to New York’s Recommended Uniform Screening Panel (RUSP) in 2014 and to the U.S. RUSP in 2016, detects elevated very long chain fatty acid levels in the blood, a clear indicator of ALD. With newborn screening, boys like Conner can be identified and treated early.
Despite the clear advantages of screening, only 35 states and Washington D.C. currently follow the recommendation to test newborns for ALD. For more information, including advocacy support, visit the ALD Alliance.
‘Our best bet for Conner’
But the family was met with more obstacles: There were no good donor matches for Conner — and their local hospital was not equipped to perform a transplant. Richelle was also worried about a serious complication of stem cell transplants: graft-versus-host disease, in which the donor stem cells react against those of the patient. Conner’s neurologist in New York referred him to Boston Children’s, hoping they could help.
They soon had an appointment with Dr. Christine Duncan, clinical director of Boston Children’s Gene Therapy Program, and Dr. Florian Eichler, director of the Center for Rare Neurological Diseases at Massachusetts General Hospital, who work together to evaluate and care for patients with CALD. Richelle immediately felt reassured. “CCALD is so rare that even most medical professionals don’t know much about it unless they’re a pediatric neurologist,” she explains. “But when we met with Drs. Duncan and Eichler, we felt like, ‘they’ve got this.’”
And — finally — there was more good news. Just weeks before, the U.S. Food and Drug Administration had approved SKYSONA (elivaldogene autotemcel) to slow the progression of neurologic dysfunction in boys ages 4 to 17 with early, active CCALD. This gene therapy is based on a study protocol developed by an international research team led by Dr. Eichler and Dr. David Williams, chief of Hematology/Oncology and founder of the Gene Therapy Program at Boston Children’s. In this approach, bone marrow is to be taken not from the donor but from the child himself, treated with a virus carrying the gene for the ALD protein, then reinfused.
“We knew this was our best bet for Conner,” says Richelle. “With the timing of everything, it just felt heaven sent.”
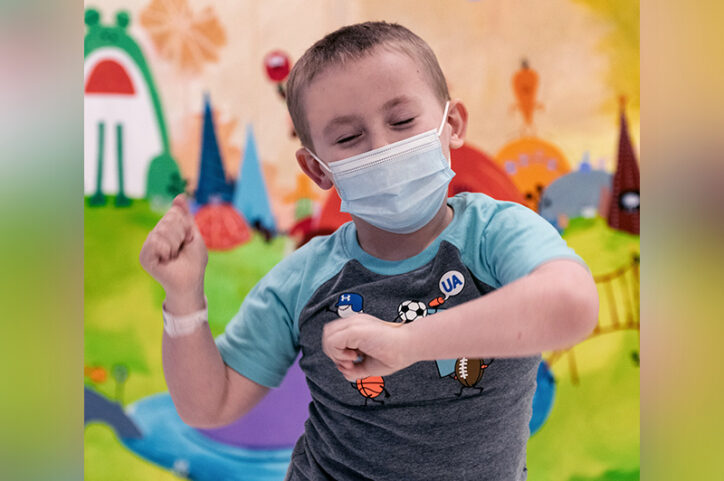
Living proof
The next six months held a flurry of paperwork, blood tests — and a lot of anxious waiting. In January 2023, Conner underwent a procedure to remove some of his stem cells, which were then processed and treated with the healthy ALD gene. In March, he returned to Boston Children’s to begin chemotherapy, which prepares the bone marrow for the transplanted stem cells. Conner was the first person in the world to receive the FDA-approved biological product.
The path hasn’t always been easy. Like a lot of kids, Conner finds doctor’s visits stressful, and his parents have had to negotiate logistical issues like insurance approvals and childcare for their older sons. But when the IV infusion of SKYSONA finally began to flow into Conner’s body, Richelle and Adam knew they had made the right decision. The therapy isn’t a quick fix: Conner will remain at Boston Children’s for three to four weeks after the infusion and will be monitored closely for the next 15 years. But if results from the clinical trials that led to SKYSONA’s approval are any indication, it will give him a chance to survive and thrive — an opportunity for which his family will be forever grateful.
“Conner is living proof,” says Richelle, “that when it seems like everything is going wrong, everything else can go right.”
Learn more about the Gene Therapy Program.
Related Posts :
-

After decades of evolution, gene therapy arrives
As early as the 1960s, scientists speculated that DNA sequences could be introduced into patients’ cells to cure genetic disorders. ...
-

Matthew, the ‘wee marvel’: One of the first ALD gene therapy recipients
When the Elliott brothers are asked how many siblings they have, they always say, “four.” It’s a way of ...
-

The space between heartache and happiness: Two sons with adrenoleukodystrophy
When Paul and Liliana Rojas talk about their life, they describe it in one of two ways — the way it ...
-

Forty years waiting for a cure: ALD gene therapy trial shows early promise
A small piece of notepaper, folded twice, sits tucked in a slot of the secretary desk in the living room. ...





