Beyond fluid buildup: Rethinking congenital hydrocephalus
Hydrocephalus is classically seen as a plumbing problem, the result of too much cerebrospinal fluid (CSF) in the brain or dysregulation of fluid flow. It is usually treated with shunts to drain the CSF, or with ETV/CPC, which makes a small hole to drain the CSF and burns off the choroid plexus tissue that produces it. Despite these treatments, some patients have poor neurodevelopmental outcomes.
“Even though we can now treat hydrocephalus endoscopically, without placing a shunt, the consequences of the initial brain injury or developmental anomaly are not reversed,” notes Benjamin Warf, MD, a neurosurgeon in the Hydrocephalus Program at Boston Children’s Hospital.
New research in Nature Neuroscience turns the “plumbing” concept on its head, at least for some children with congenital hydrocephalus. It suggests that as many as 1 in 4 congenital cases are genetic, and that the enlarged brain ventricles that mark hydrocephalus can result not from fluid overaccumulation, but from less brain being formed during fetal development.
“This form of congenital hydrocephalus falls under a radically different paradigm: genetic dysregulation of neural stem cell growth in the brain,” says study leader Kristopher Kahle, MD, PhD, who holds research appointments in Neurosurgery and Genetics and Genomics at Boston Children’s and is chief of Pediatric Neurosurgery at Massachusetts General Hospital. “When we see enlarged ventricles, the classic thinking is that they reflect too much fluid in the brain. Our study shows that they can reflect brain development that is severely compromised.”
Patients with these genetic mutations may benefit from approaches that optimize neurodevelopment rather than simply draining CSF. Potentially, targeted drugs, gene editing, or in utero gene therapies could help correct brain development and prevent hydrocephalus, possibly without neurosurgery.
Turning hydrocephalus on its head
Kahle, first author Duy Phan, PhD, an MD/PhD student in the Kahle lab, and other colleagues studied 483 patients with congenital hydrocephalus with whole-exome sequencing. They also sequenced many of the patients’ parents, enabling them to identify de novo mutations occurring after conception.
This study describes the latest of a series of neurodevelopmental disorders that can have multiple genetic causes, including cerebral palsy, epilepsy, and other unexplained conditions.
Through these efforts, they found 93 different genes associated with hydrocephalus. “We then localized the affected genes’ function in time and space,” says Kahle.
To do this, the team used single-cell RNA sequencing and consulted atlases of gene transcription in the brain to determine where and at what point in brain development the mutated genes turn on.
The genes’ expression converged in neuroepithelial cells, the earliest-forming neural stem cells. These cells line the embryonic cerebral ventricles in the neural tube during the first several weeks of brain development, surrounding the developing fluid space.
“This epithelium is critical for populating the rest of the brain with neurons and glia,” says Kahle.
Too few brain cells
The mutations were most commonly in TRIM71, a gene that regulates neural stem cell growth and differentiation. When the team modeled the mutations in mice, the animals developed hydrocephalus, but without showing defects in the flow or reabsorption of CSF.
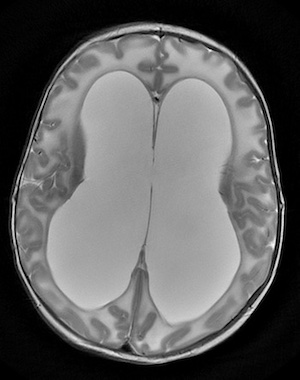
Further studies revealed that the stem cells in the neuroepithelium differentiated too soon in embryonic development. Cell proliferation was decreased, with fewer neurons than would be expected. This led to an underdeveloped cortex.
“Perhaps most notably, this underdevelopment results in a ‘floppy’ cortex that is unable to hold the pressure exerted by fluid in the ventricles,” says Phan. “Thus, fluid passively accumulates and distends the ventricles even when there is no anatomical obstruction to CSF flow.”
A clinical agenda
Kahle and Phan plan to continue genetic sequencing in patients with hydrocephalus and correlate the results with clinical findings, in collaboration with Christopher Walsh, MD, PhD, chief of Genetics and Genomics at Boston Children’s and an expert in neurodevelopmental genetics.
“Experience always shows that the more we learn about underlying mechanisms, the closer we come to individualizing treatment approaches,” says Walsh. “This work suggests that we should consider genetic testing for a larger slice of patients with hydrocephalus than we might have suspected previously.”
Kahle and Phan will also compare the genetic data with fetal and infant neuroimaging studies to establish a diagnostic MRI “signature” for genetic hydrocephalus. Finally, in collaboration with Boston Children’s neurosurgeons, including Warf, Scellig Stone, MD, PhD, and Mark Proctor, MD, they will seek to correlate genetic profiles with patients’ outcomes after receiving shunts or ETV/CPC.
“There are scattered reports of kids not improving after being shunted, and ventricular size not changing,” Kahle says. “Some patients may benefit from more conservative management.”
Hydrocephalus is the final common pathway of many etiologies, notes Warf.
“Understanding more about the various underlying mechanisms of hydrocephalus will lead to therapeutic advances that may not only improve outcomes, but perhaps even prevent hydrocephalus from developing,” Warf says.
Contact the Hydrocephalus Program at Boston Children’s.
Related Posts :
-

Rethinking the origins of cerebral palsy
Cerebral palsy (CP) has widely been viewed as the result of perinatal oxygen deprivation or other birth-related factors like prematurity. ...
-

Diving deep on epilepsy genetics
When child neurologist Annapurna Poduri, MD, MPH finished her clinical epilepsy fellowship at Boston Children’s Hospital in 2004, she was ...
-

Solving neurodevelopmental mysteries, one gene, one child at a time
Suheil Day was born early, at 37 weeks. Aside from a slight head lag and mild muscle weakness, nothing seemed terribly ...
-

Sudden, unexplained child deaths often have a genetic cause
When a baby or toddler dies without warning, parents often blame themselves. A study at Boston Children’s may provide ...





