Precision drug delivery systems could ‘trigger’ an age of nanomedicine
What if we could deliver biocompatible nanoparticles into the body and then activate them to release drugs exactly where they are needed, without causing side effects elsewhere?
Scientists like Daniel Kohane, MD, PhD, of Boston Children’s Hospital, are developing nanoscale drug delivery systems to do just that, using a variety of materials and triggers that are sensitive to a range of specific stimuli.
“Triggerable drug delivery systems could improve the treatment of many diseases by reducing side effects and increasing the effectiveness of therapeutics,” says Kohane, who directs the Laboratory for Biomaterials and Drug Delivery at Boston Children’s. He is the senior author on a recent article about the topic in Nature Reviews Materials.
One potential use of nanoscale drug delivery systems is of special interest to Kohane and his lab members, who are part of the Department of Anesthesiology, Perioperative and Pain Medicine Research at Boston Children’s.
“The ability to toggle ‘on’ or ‘off’ targeted delivery of painkillers would be very advantageous for pain management,” says Kohane.
Kohane and his team envision an injectable material that could be delivered to sites of the body causing pain. Once injected, the material, which acts like a drug depot, could be triggered by external application of low-intensity, near-infrared light (NIR).
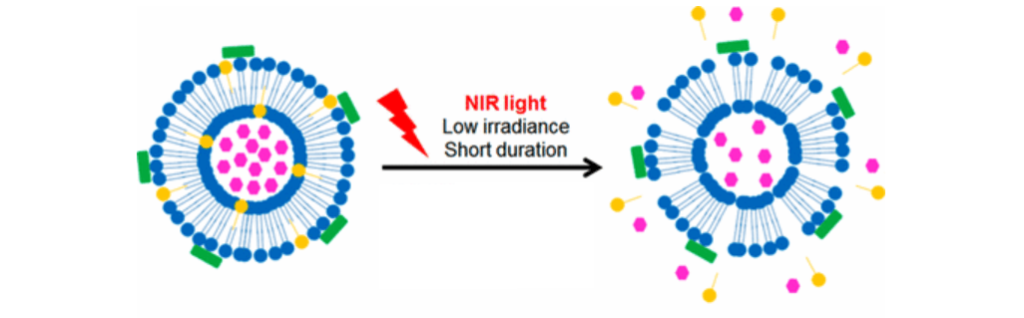
The on-demand system comprises biocompatible gold nanorods and liposomes, which are sphere-shaped tiny bubbles. When heated by the light, the gold bits trigger the liposome bubbles to release pain-blocking drugs, providing local anesthesia. In early laboratory models, the technique has already shown to be quite effective.
But this is just one of seemingly countless ways to design externally-triggerable drug delivery systems.
Configurable drug delivery systems
Kohane’s lab is utilizing several different triggers and triggerable materials to develop local and systemic delivery approaches. In addition to near-infrared light, several other light sources could make suitable triggers, including light-emitting diodes (LEDs), lasers and UV-visible light. What’s more, radiation, magnetic fields, static fields, ultrasound, electrical pulses, pH levels and certain chemicals can all be manipulated and utilized as triggers for different purposes.
In fact, some drug delivery systems are even engineered to work with more than one trigger, either external or internal, to enrich drug localization.
“A key concept of drug delivery systems is that they can be delivered systemically, but triggered locally,” says Yanfei Wang, PhD, a research fellow in Kohane’s lab and co-author of the recent Nature Materials Reviews.
In this fashion, systemically-delivered nanoparticles could circulate through the body, adhering to specific disease sites and concentrating their therapeutic payload there.
But for drug delivery systems to move out of the lab and into the clinic, more research is needed to vet safe, effective techniques that are simple and inexpensive to build and trigger.
With any of these approaches, drug loading, release kinetics, nanoparticle degradation and other factors must considered during the design process, say Kohane and Wang. As of now, some materials still need to be evaluated to see how they will interact with the body.
“Biocompatibility is extremely important, naturally,” says Kohane. “The goal of drug delivery systems is to prevent side effects, not add new ones.”
Related Posts :
-

The people and advancements behind 75 years of Boston Children’s Cardiology
Boston Children’s Department of Cardiology has more than 100 pediatric and adult cardiologists, over 40 clinical fellows learning the ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity and ...
-

Naloxone on demand: Shining a light to reverse opioid overdose
Overdose deaths from fentanyl and other opioids are at record highs in the U.S. Naloxone, if delivered soon after ...
-

Gold particles and light could melt venous malformations away
Venous malformations — tissues made up largely of abnormally shaped veins — are often difficult to treat, especially when located in sensitive ...