A simpler way to measure complex biochemical interactions
Life teems with interactions. Proteins bind. Bonds form between atoms, and break. Enzymes cut. Drugs attach to cell receptors. DNA hybridizes. Those interactions make the processes of life work, and capturing them has led to many medical advances.
“Determining which molecules interact, and measuring the strength of these interactions is fundamental for many areas of research, from drug discovery to understanding the mechanisms underlying disease,” says Wesley P. Wong, PhD, a biophysicist with Boston Children’s Hospital’s Program in Cellular and Molecular Medicine (PCMM), Harvard Medical School and the Wyss Institute for Biologically Inspired Engineering.
Technologies abound for studying molecular-level interactions quantitatively. But most are complex and expensive, requiring dedicated instruments and specific training on how to prep samples and run the experiments.
Wong and his team, including graduate student Mounir Koussa and postdoctoral fellows Ken Halvorsen, PhD (now at the RNA Institute) and Andrew Ward, PhD, have created an alternative method that democratizes the process. Using electrophoresis gels, found in just about any biomedical laboratory, they’ve developed what they call DNA nanoswitches. These switches let researchers make interaction measurements without complex instruments, at a cost of pennies per sample.
Binding and bending
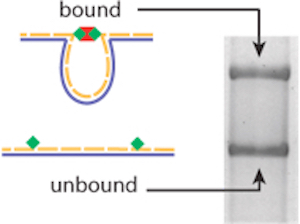
As Wong and his team report in Nature Methods, the nanoswitches start with a single-stranded DNA “scaffold” made in a laboratory. Those scaffolds are mixed with “staple” strands—oligonucleotides that complement the scaffold—that are tagged with the interacting molecules a researcher wants to study.
So, for instance, to see how long it takes for a cell receptor and a ligand molecule to bind then separate (or dissociate)—a surrogate for the strength of their interaction—a researcher first attaches the ligand molecules to staple strands and mixes them with a scaffold strand. Because they’re made of complementary nucleotide sequences, the scaffolds and ligand-bound staples then assemble themselves into DNA nanoswitches.
Next, the researcher adds her receptors to the mix. As the receptors and ligands bind, the nanoswitches fold into closed loops. At different time points, she then takes a sample of the mixture and runs it through a gel.
“By watching how the nanoswitches transition from open to closed or closed to open over time, we can characterize not only the strength of [an] interaction but also its kinetic properties,” Wong explains in this video produced by the Wyss Institute:
Wong, who recently won a Beckman Young Investigator Award from the Arnold and Mabel Beckman Foundation, says the technology can go far beyond this simple example, allowing scientists to easily study complex multicomponent interactions and opening up new areas of research.
“Many things in biology aren’t just two molecules coming together, but rather multiple components interacting,” Wong says. “With the nanoswitches, we can get a distinct signal for each interaction in a system,” all in the same experiment and on the same gel.
Bringing high-end tech within reach
The DNA nanoswitch technology is just one way Wong aims to help a broader range of laboratories run highly complex experimental studies. Take measuring and manipulating single molecules. Such measurements require sophisticated, costly equipment, and typically only one measurement can be taken at a time.
Five years ago, while at the Rowland Institute at Harvard University, Wong and Halvorsen developed a simple, high-throughput alternative method using a standard laboratory centrifuge modified with a rapidly rotating microscope. Called a centrifuge force microscope, the adapted tool lets researchers run single-molecule tests on many individual molecules simultaneously.
“I love the process of taking methods that have potential for wider impact in science and adapting them so that everyone can make these measurements in their own labs,” Wong says. “With the DNA nanoswitches, we hope we’ve created a measurement method that’s inexpensive and simple for people to use, using materials they largely already have.”
The Wong laboratory is making starter kits available to researchers and educators interested in trying the DNA nanoswitch technology. To start using DNA nanoswitches in your laboratory or class, request a starter kit and view a series of tutorial videos from the Wyss Institute.
Learn more in this press release issued by the Wyss Institute.
Related Posts :
-

Building better antibodies, curbing autoimmunity: New insights on B cells
When we’re vaccinated or exposed to an infection, our B cells spring into action, churning out antibodies that are ...
-

Exposing a tumor’s antigens to enhance immunotherapy
Successful immunotherapy for cancer involves activating a person’s own T cells to attack the tumor. But some tumors have ...
-

EEG markers in early life could help predict and diagnose anxiety
Anxiety disorders are the most common mental health problem among children and adolescents and are a risk factor for adult ...
-

Could the right dietary fat help boost platelet counts?
Aside from transfusions, there currently is no way to boost people’s platelet counts, leaving them at risk for uncontrolled ...