New angles for blocking Shiga and ricin toxins, and new light on an iconic biological process
Min Dong, PhD, and his lab are world experts in toxins and how to combat them. They’ve figured out how Clostridium difficile’s most potent toxin gets into cells and zeroed in on the first new botulinum toxin identified since 1969. Now, they’ve set their sights on Shiga and ricin toxins, and not only identified new potential lines of defense, but also shed new light on a fundamental part of cell biology: glycosylation.
The study, published in PLOS Biology last week, used CRISPR/Cas9 technology to explore what factors in cells are necessary for the toxins to get in. Shiga toxin, a widespread source of food poisoning, is produced by Shigella dysenteriae and some E. coli strains such as O157:H7. It occasionally causes hemolytic uremic syndrome, a serious kidney condition, in children. Ricin is a plant toxin used as a bioterrorism agent.
What do toxins need in a cell receptor?
Once inside our tissues, Shiga and ricin toxins work similarly, disrupting cells’ ability to make proteins. To get in, though, they rely on different entry portals. Shiga toxins use a type of glycolipid (a fatty molecule with an attached sugar) called Gb3 as their receptor, while ricins use a variety of glycans (sugar molecules).
But beyond that, little was known. Dong and first author Songhai Tian, PhD, researchers in Boston Children’s Hospital’s Department of Urology, hoped to complete the picture.
The research team began with Shiga toxins. They used CRISPR/Cas9 to conduct a genome-wide screen. This entailed systematically deleting genes one by one in a cell line, to see if the loss of any of them prevented the toxins from entering.
“This was not possible previously, because the receptor for the toxin isn’t found in most cell types,” says Dong. “Songhai screened many different cell lines without much success, until he talked to our neighbor, Dr. Rosalyn Adam in the Department of Urology, who happens to have a few bladder cancer cell lines. One of these turns out to be super sensitive to Shiga toxins because it has a high level of Gb3. Finding a good cell model was the first breakthrough in the project.”
The second screen, in readily available HeLa cell lines, looked for cell factors required for ricin toxicity.
“The screen approach was very powerful,” says Tian. “It identified almost all the known factors for both toxins, as well as some novel factors.”
New leads on blocking toxicity
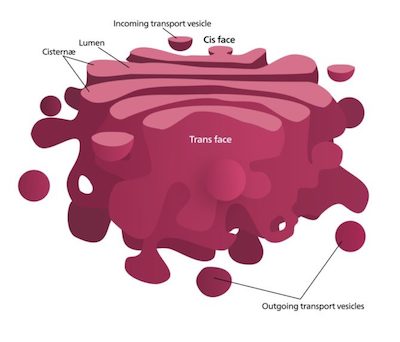
When the team compared the two screens, they found two factors that both Shiga and ricin toxins require to penetrate cells: the transmembrane proteins TMEM165 and TM9SF2. Both are found in the cell’s Golgi apparatus, whose job it is to modify and package large molecules made by the cell. When either protein was deleted, the cells produced reduced amounts of a group of fatty molecules called glycosphingolipids, including Gb3.
TMEM165 and TM9SF2 are too general in their function to serve as safe targets to try to block with drugs, Dong says, but they could point the way to other targetable molecules.
A third protein, LATMP4a, offers more direct promise. It is specifically required for cells to make Gb3, the receptor used by Shiga toxin. “This protein was not known before, and we think it would make a very good therapeutic target for Shiga toxicity,” says Dong.
Updating the textbook chapter on glycosylation
Dong is equally excited about what the work has to tell us about glycosylation — the attachment of sugars to large molecules like proteins and lipids. This fundamental biologic process enables our cells to create highly varied molecules with diverse functions, above and beyond what’s encoded in our genes. It also has a role in disease.
“We used toxins as a probe to understand a key cellular process,” Dong elaborates. “The two new Golgi proteins identified in our screens, TMEM165 and TM9SF2, likely regulate the overall environment in the Golgi apparatus. We’re now trying to understand what they’re regulating specifically.”
This isn’t the first time one of Dong’s toxin investigations turned up something unexpected. His team’s recent work on C. difficile’s toxin B revealed a potential anti-cancer strategy.
The current study was funded by the National Institutes of Health, the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center, Intelligence Advanced Research Projects Activity (IARPA) and the Burroughs Wellcome Fund. See the paper for a full list of authors and affiliations.
Related Posts :
-

Building better antibodies, curbing autoimmunity: New insights on B cells
When we’re vaccinated or exposed to an infection, our B cells spring into action, churning out antibodies that are ...
-

Exposing a tumor’s antigens to enhance immunotherapy
Successful immunotherapy for cancer involves activating a person’s own T cells to attack the tumor. But some tumors have ...
-

Could a GI bug’s toxin curb hard-to-treat breast cancer?
Clostridium difficile can cause devastating inflammatory gastrointestinal infections, with much of the damage inflicted by a toxin the bug produces. ...
-

A new approach to C. diff? Targeting the inflammation, not the bacteria
Clostridium difficile (C. diff) intestinal infections can cause severe, debilitating diarrhea in patients who are hospitalized or on immunosuppressive therapies. ...