Natural killer cells: Protect the placenta cell but kill the infection
Infections that reach the placenta can lead to spontaneous abortions, intrauterine growth restriction, congenital abnormalities, and premature births. New research from the laboratory of Judy Lieberman, MD, PhD, and colleagues at Harvard University shows that a group of cells near the site where the placenta attaches to the uterine wall plays an essential role in preventing these infections. The study also sheds light on how the mother’s body tolerates the fetus, which is like a foreign tissue.
Key takeaways
- This is the first report that immune cells can destroy bacteria inside a cell without killing the cell.
- Maternal natural killer cells sprout tiny tubes to transfer bacteria-killing granulysin to fetal cells.
- Granulysin protects pregnant mice with bacterial infection from miscarriage.
- The findings provide an early look into mechanisms preventing fetal infection and spontaneous abortion.
In a study published in Cell, Lieberman’s laboratory in the Boston Children’s Hospital Program in Cellular and Molecular Medicine found that natural killer (NK) cells on the mother’s side of the placenta, known as the decidua, can kill bacteria inside placental cells. And they can do so without killing the cell — an immune cell achievement not known before this study.

To achieve this feat, the decidual NK cells inject an anti-microbial protein called granulysin into the cytoplasm of infected placental cells, the study found. In earlier research, the Lieberman lab learned that granulysin could kill bacteria, fungi, and parasites.
“In human cell studies and in live mice, we found that granulysin can control infections of the fetus and that it does so without harming the infected fetal cells,” says Lieberman.
An abundance of NK cells in the placenta
In the first trimester of pregnancy, NK cells comprise about one-third of all the maternal cells in the decidua. These cells are in direct contact with fetal cells where the placenta is anchored to the uterus. Their abundance at this vulnerable location led Lieberman’s team to rethink their possible role in fighting infections and whether granulysin, which these NK cells produce at high levels, might be involved.
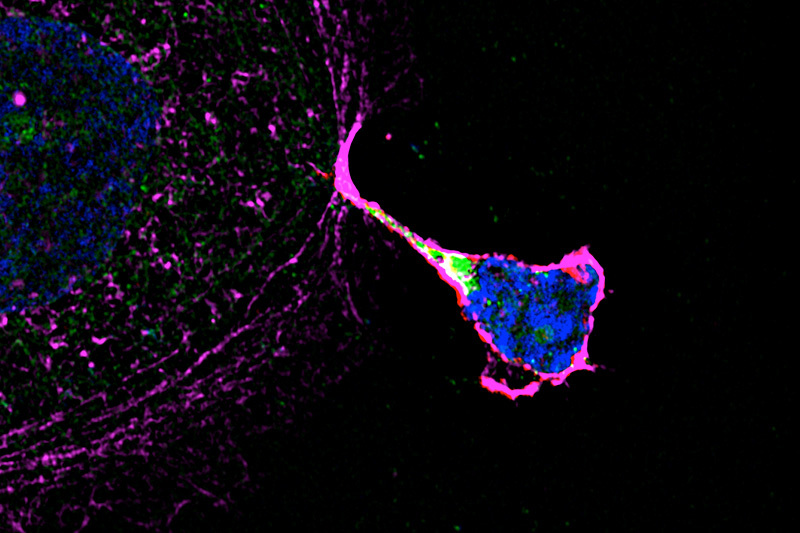
Investigations revealed that decidual NK cells sprout “nanotubes” that transfer the contents of their cytoplasm — including granulysin — into the fetal placental cell. Once in the cell, granulysin finds its way to intracellular bacteria, binds to their cell membranes, and pokes holes in them to kill the bacteria. The fetal placental cell is not killed because granulysin does not harm its cell membrane.
Understanding a new immune defense that can protect against infection in the placenta without harming fetal tissues may point the way to new ways to protect the fetus.”
– Angela Crespo

While the nanotubes inject granulysin, they do not transfer other molecules in NK that can harm fetal placental cells, allowing the body to both attack an infection but tolerate the presence of foreign tissue of the placenta and fetus.
“This phenomenon adds another piece to the puzzle of maternal immune tolerance of the fetus, which has always been regarded as a paradox,” says co-first author Angela Crespo, PhD, research fellow in the Lieberman lab. “Decidual NK cells can fight an infection in the fetus while at the same time maintaining tolerance to the fetus, allowing for normal fetal development.”
Preventing spontaneous abortions
The team used mice genetically engineered to produce granulysin to see how the NK cells respond to infection in a living animal.
“When we infected these mice with Listeria bacteria during pregnancy, they could carry their pregnancies to term, whereas without granulysin, they will abort,” says Lieberman. “That is a pretty potent immune defense.”
While this research was done only in mice, it provides insight into the mechanisms of maternal-fetal tolerance and immune defense, as well as prevention of infection transmission from mother to fetus.
“Understanding a new immune defense that can protect against infection in the placenta without harming fetal tissues may point the way to new ways to protect the fetus,” says Crespo. “This would be very valuable to improve the current standard of care for pregnant women since it is very common that congenital infections go undetected, even after spontaneous abortion.”
Do other cells in the body use this defense system?
Lieberman’s lab plans on studying how the nanotubes form and what else might be transferred to fetal cells besides granulysin.
“For a long time, people thought that natural killer cells in the placenta were important for regulating the blood supply to the placenta,” says Lieberman. “So it is possible that other molecules are being transferred to the fetal cells, like nutrients or growth factors or any number of things that might help the fetal cells form the placenta.”
As in the placenta, the immune system is constrained in other areas of the body — namely the eyes and testes — to prevent damage to these organs.
“We would also like to know if this phenomenon is important in these and other sites and which cells can do it,” says Lieberman. “We also want to understand what infections NK cells and granulysin may protect against.”
Crespo and Sachin Mulik (formerly of Boston Children’s, now the University of Texas Health Science Center) are co-first authors. Corresponding authors are Lieberman, Tamara Tillburgs, (formerly of Harvard University, now at Cincinnati Children’s Hospital), and Jack Strominger of Harvard University. Other contributors are Farokh Dotiwala (formerly of Boston Childrens, now at The Wistar Institute), James Ansara, Sumit Sen Santara, Kayleigh Ingersoll, Cristian Ovies, and Caroline Junqueira of Boston Children’s. This work was funded by the National Institutes of Health.
Read more about discoveries made in the Program in Cellular and Molecular Medicine.
Related Posts :
-

Finding comfort and answers for twin-twin transfusion syndrome: Shannon’s story
Shannon’s journey through a challenging pregnancy with TTTS (twin-twin transfusion syndrome) was, as she puts it, an emotional rollercoaster. ...
-

Building better antibodies, curbing autoimmunity: New insights on B cells
When we’re vaccinated or exposed to an infection, our B cells spring into action, churning out antibodies that are ...
-

Could SIDS be caused by unrecognized brain infections?
Some infants who pass away from sudden infant death syndrome (SIDS) are known to have had acute minor infections. Could ...
-

Exposing a tumor’s antigens to enhance immunotherapy
Successful immunotherapy for cancer involves activating a person’s own T cells to attack the tumor. But some tumors have ...