A master regulator of kidney health?
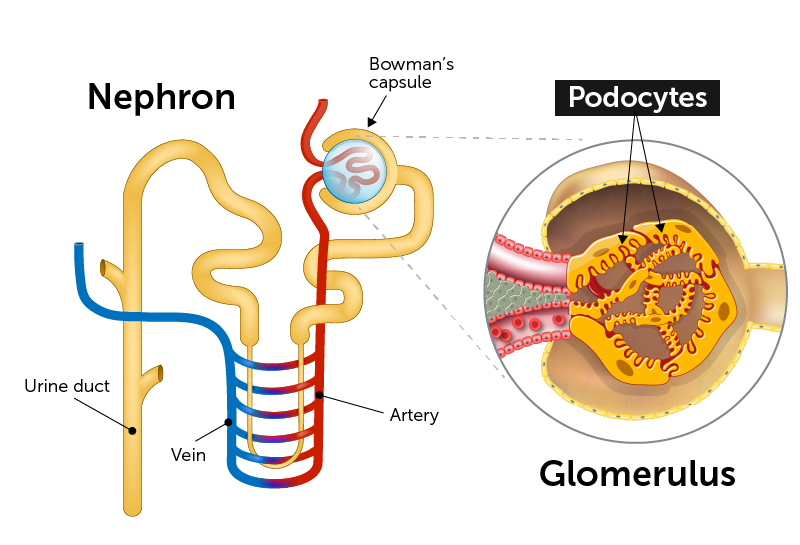
End-stage kidney disease often begins with injury to podocytes. These highly specialized cells are a critical part of the glomeruli, clusters of capillaries that serve as the filtration units in our kidneys’ tightly-packed nephrons. As their name suggests, podocytes extend tiny foot processes to intermingle with the capillaries and filter the blood, maintaining the proper water and salt balance.
Injury to the blood-filtering cells known as podocytes can cause kidney failure. This study finds a master genetic program that triggers self-repair and could point to possible treatment targets.
In many kidney diseases, the foot processes become damaged and the podocytes themselves detach from the capillaries. The resulting loss of filtration capacity can be life threatening. More than 100,000 Americans end up on dialysis each year for lack of any other effective treatment. African Americans are disproportionately affected.
The lab of Jordan Kreidberg, MD, PhD, in the Boston Children’s Hospital departments of urology and nephrology, has been trying to understand how kidneys and podocytes naturally maintain themselves. In a study today in Science Advances, the team describes a master genetic program that seems to orchestrate podocytes’ innate injury-repair response.
“Understanding this network of genes is a major step towards identifying pathways that might be targeted therapeutically,” says Kreidberg.
At the heart of the network is a protein called Wilms tumor-1 (WT-1). Known as a transcription factor, WT-1 interacts with multiple genes, regulating which transcribe their DNA and are therefore are expressed or turned “on.” (Kreidberg and his colleagues previously showed that if you knock out WT-1 in a mouse, the kidneys never develop.)
A natural repair program for podocytes
Kreidberg’s team worked with two models to learn what happens genetically after podocyte injury: a mouse model and human kidney organoids with mini-nephrons, created from stem cells.
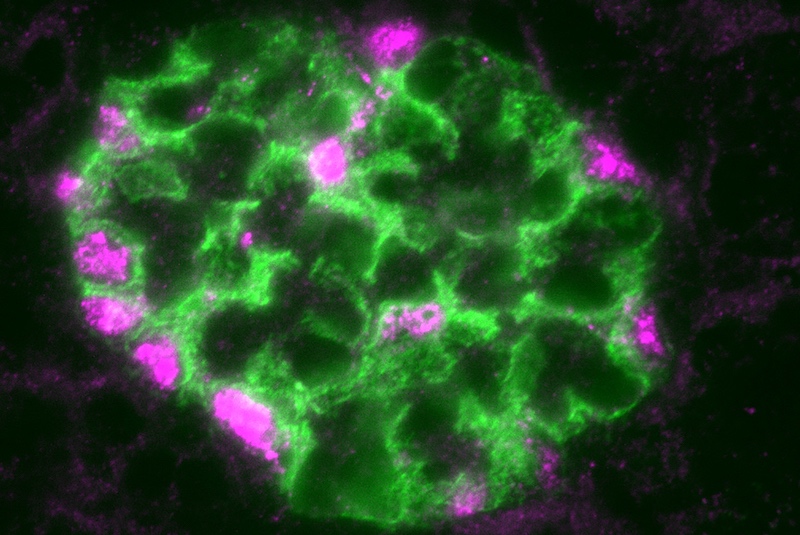
They found that, after injury, WT-1 levels rose. This led to activation of other transcription factors and changes in the expression of multiple genes known to be important in maintaining the kidney’s filtration barrier. (Included is the gene for nephrin, shown above.) Of the more than 1,200 genes that WT-1 bound to, 223 at least doubled their expression levels.
“Unfortunately, and for reasons we don’t yet fully understand, expression of WT-1 ultimately decreases, and you end up with poorly functioning kidneys,” says Kreidberg. “Our studies don’t yet point to a particular target, but if there were a way to sustain WT-1 expression, we envision that this might protect podocytes and avoid kidney failure.”
Kreidberg and his colleagues continue to study WT-1 and other transcription factors involved in the podocyte repair process. While there aren’t yet specific targets for drugs, the investigations could potentially lead to better treatments for focal segmental glomerulosclerosis (FSGS), a severe form of nephrotic syndrome, as well as kidney failure due to diabetes or hypertension, Kreidberg says.
A rare and hard-to-study cell
The study also marks a significant technical accomplishment.
“It’s only in the last few years that we’ve been able to get podocytes out of the glomerulus and do gene expression studies on them,” Kreidberg notes. “Glomeruli make up just 1 percent of the mass of the kidney.”
Kreidberg and Peter J. Park of Harvard Medical School’s Department of Biomedical Informatics were co-corresponding authors on the study. Sandrine Ettou of the Kreidberg lab and Youngsook Lucy Jung of the Park lab were co-first authors. The organoid work was a collaboration with the lab of Ryuji Morizane of Massachusetts General Hospital. The National Institutes of Health and the Uehara Memorial Foundation funded the study.
Learn about other nephrology research, urology research and the Division of Nephrology.
Related Posts :
-

‘Empowered to be there for Teagan’: New parents learn about hearing loss
Teagan O’Brien is a bright, spunky 4-year-old who loves reading, dancing, and playing outdoors. Her parents, Kim and Donnie, ...
-

Building better antibodies, curbing autoimmunity: New insights on B cells
When we’re vaccinated or exposed to an infection, our B cells spring into action, churning out antibodies that are ...
-

A journey through the intestine during colitis, cell by cell
Inflammatory bowel disease (IBD), causing devastating abdominal pain, persistent diarrhea, and rectal bleeding, is hard to control with current treatments. ...
-

Genetic variants are found in two types of strabismus, sparking hope for future treatment
Determining how genetics contribute to common forms of strabismus has been a challenge for researchers. Small discoveries are ...