S1P and its receptor: New approaches to cancer?
In 1998, when Timothy Hla, PhD, and his colleagues identified and cloned the receptor for sphingosine-1-phosphate (S1P), it generated a lot of excitement. S1P, a lipid originally discovered in the 1960s, was known to play various roles in the body and in disease. But it wasn’t thought that lipids could have receptors, and it wasn’t fully appreciated that they could send messages into cells.
“We were very busy for the next 10 years,” says Hla. “S1P biology has become quite a large field.”
Hla heads a lab in Boston Children’s Hospital’s Vascular Biology Program, so among the group’s questions was what S1P does in the vascular system. In the early 2000s, Hla and colleagues showed that S1P is essential for angiogenesis, or the growth of blood vessels, finding that endothelial cells, which line blood vessels, are rich in the S1P receptor. When they blocked the S1P pathway in animal models, embryos could not form blood vessels and died.
“Then, a cool serendipitous thing happened in the S1P field,” Hla says.
S1P: From the vascular system to the immune system and back
Biochemists in the pharmaceutical industry, seeking new drugs for autoimmune disease and transplant rejection, were studying a compound derived from a fungus, used in Chinese medicine. The drug seemed to prevent T cells from attacking transplants or the body’s own tissue. The biochemists learned of Hla’s work and realized that their compound interacted with the S1P receptor Hla’s team had characterized.
“The same receptor I worked on in endothelial cells is also in T cells, B cells, and other immune cells,” Hla says. “It turns out that T cells need S1P to circulate in the body.”
With the help of Hla’s research, the first S1P inhibitor, fingolimod, was approved in 2010 for multiple sclerosis. By blocking the S1P receptor, the drug prevents T cells from leaving the lymph nodes to attack the spinal cord and brain, the hallmark of multiple sclerosis. (Other S1P inhibitors have since been approved.)
For his part, Hla realized that fingolimod could be a valuable tool for exploring S1P’s role in the body and how it works. For the past decade, his lab has done just that, enabling the development of other drugs in this pathway to treat various diseases.
Making vessels better drug carriers
Hla, research fellow Andreane Cartier, PhD, and colleagues focused on cancer in a recent study published in PNAS. They knew that tumor progression depends upon angiogenesis, and that S1P and its receptor are essential in blood vessel growth.

Putting these ideas together, they genetically manipulated mice with lung cancer, melanoma, and breast cancer, creating two test conditions. In one, the animals’ tumor blood vessels had no S1P receptors; in the other, the vessels had extra S1P receptors.
Blood vessels in tumors tend to be tortuous and leaky. And in mice lacking S1P receptors, the vessels grew even more chaotically.
“They were a big mess, convoluted, with poor blood flow,” says Hla.
Enhancing cancer therapy with S1P
Conversely, when tumor blood vessels had more S1P receptors, allowing S1P to send its signal, the vessels became less tortuous, less leaky, and more like normal vessels.
“That’s a good thing,” says Hla, “because if you want to treat cancers with chemotherapy, you need good conduits. S1P coming from the circulation makes the vessel networks more functional. It’s basically sculpting them to make nice channels.”
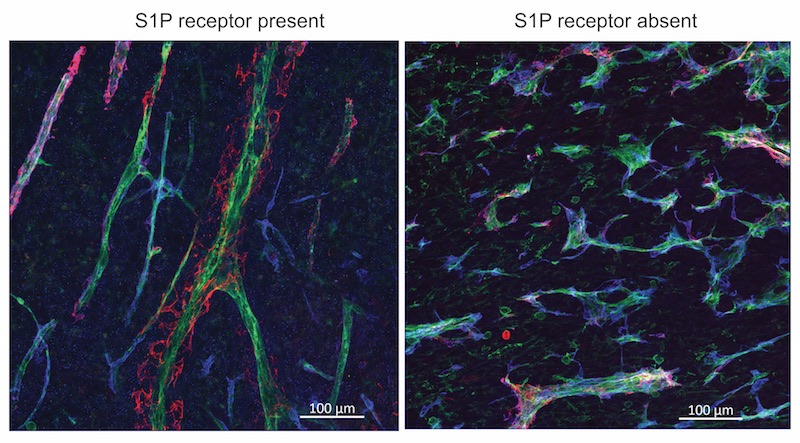
Once the vessels were normalized by boosting S1P receptors, chemotherapy or immune checkpoint inhibitor drugs indeed shrunk tumors more effectively. In contrast, mice whose tumor vessels lacked the receptor had larger tumors and enhanced metastasis.
What’s next?
Hla’s team is now looking for compounds that could be given to boost S1P receptors so that cancer drugs can better get to tumors. They are also revisiting S1P’s role in the immune system, looking at ways to harness S1P and its receptor to enhance cancer immunotherapy. Such an approach could help mobilize T cells to attack tumors. “Immune cells must use S1P to be able get to the right destination,” Hla says.
Read more in this recent review article in Science by Hla and Cartier.
Andreane Cartier, of Boston Children’s Vascular Biology Program, was first author on the PNAS paper. Coauthors were Tani Leigh of Boston Children’s and Catherine H. Liu of Weill Cornell Medicine. The work was supported by National Institutes of Health (HL89934, HL117798, R35 HL135821); a Leducq Foundation Transatlantic Network Grant (SphingoNet); and a fellowship from the American Heart Association (18POST33990452).
More recent research from the Vascular Biology Program
Related Posts :
-

Building better antibodies, curbing autoimmunity: New insights on B cells
When we’re vaccinated or exposed to an infection, our B cells spring into action, churning out antibodies that are ...
-

Exposing a tumor’s antigens to enhance immunotherapy
Successful immunotherapy for cancer involves activating a person’s own T cells to attack the tumor. But some tumors have ...
-

Combining CAR-T cells and inhibitor drugs for high-risk neuroblastoma
Chimeric antigen receptor (CAR)-T cell therapy is a potent emerging weapon against cancer, altering patients’ T cells so they ...
-

Could a GI bug’s toxin curb hard-to-treat breast cancer?
Clostridium difficile can cause devastating inflammatory gastrointestinal infections, with much of the damage inflicted by a toxin the bug produces. ...