In high-risk neuroblastoma, two stem cell transplants may be better than one
Since the early 1990s, chemotherapy followed by an autologous stem cell transplant has been the standard of care for high-risk neuroblastoma, a childhood cancer that starts in nerve cells outside the brain, especially in the tissues of the adrenal glands, and sometimes the neck, chest, or pelvis. Before children receive chemotherapy to destroy the neuroblastoma, some of their healthy blood stem cells are removed and stored. These cells are later returned, helping the child weather the toxic effects of the chemo.
Yet survival has remained low: mortality in high-risk neuroblastoma can be as high as 50 percent, and nearly 70 percent of all children with neuroblastoma already have metastatic disease at diagnosis. Overall, neuroblastoma accounts for 10 to 12 percent of all child cancer deaths.
A study published last week in JAMA supports a new, even more intensive approach for most children with high-risk neuroblastoma: giving not one but two autologous stem cell transplants. In a multicenter randomized trial led by investigators at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and Seattle Children’s Hospital, the double transplant enabled children to survive longer without new or recurrent disease over three years of follow-up.
Introducing double stem cell transplant
Dana-Farber/Boston Children’s researchers pioneered the idea of tandem stem cell transplants for neuroblastoma in 1994. First, to make the approach feasible, they developed a new technique for harvesting circulating blood stem cells from very small children.

Over the course of a decade, Lisa Diller, MD and her colleagues tested and refined the double-transplant treatment. Their promising results led the national Children’s Oncology Group to open a randomized trial in 2007 (ANBL0532) to definitively compare two transplants, given roughly six weeks apart, versus one.
The Phase 3 trial initially enrolled 652 patients with high-risk neuroblastoma, averaging about 3 years of age at diagnosis. Patients first underwent surgery and six cycles of high-dose chemotherapy. After completing this phase, 355 patients were eligible for and consented to enter the next phase — which randomly assigned them to receive either two autologous stem cell transplants or just one. Radiation therapy followed all transplants.
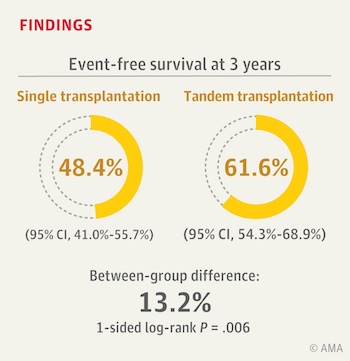
Three-year event-free survival — meaning survival without relapse, progressive disease, or a second malignancy — was significantly better in the double-transplant group (62 vs. 48 percent). About 70 percent of randomized patients went on to receive targeted immunotherapy, now a standard treatment after transplant. Within this subgroup, patients receiving two stem cell transplants versus just one also had better three-year event-free survival (73 vs. 55 percent).
Diller and her colleagues caution that the findings may not be representative of all patients with high-risk neuroblastoma. Nearly half of the original cohort ultimately wasn’t randomized to double versus single transplant. So there might be patients who don’t benefit from tandem transplant that were underrepresented in the study. The researchers also note that overall survival was not statistically significantly different between the tandem- and single-transplant groups, and that the benefit of tandem transplant might not extend to patients treated with different chemotherapy regimens before transplant.
A challenging treatment
The team presented initial results of the trial at the American Society of Clinical Oncology annual meeting in 2016. Since then, tandem transplant has been considered the standard of care for ongoing and future Children’s Oncology Group Trials.
Diller, who is Dana-Farber/Boston Children’s chief medical officer, notes that neuroblastoma therapy is a challenging ordeal for children. Treatment includes intensive chemotherapy, aggressive surgeries, double transplant, post-transplant radiation, and immunotherapy.
Emily Coughlin was one of the patients in the trial. She was in the intensive care unit twice during the first transplant; luckily, the second one was a little easier. She has suffered side effects — including loss of kidney function and hearing loss — but now enjoys a good quality of life.
Diller hopes for even better outcomes in the future.
“As encouraging these new tools are, the fact remains that we are treating some of our youngest patients with the most toxic therapies in our arsenal,” she says. “While we continue to strive to improve survival, we must also seek ways to reduce the toxicity of our treatments.”
Diller was the study’s senior investigator. Julie Park, MD, of Seattle Children’s Hospital, was the paper’s first author. The study was supported by the National Institutes of Health, the National Cancer Institute (grant to Children’s Oncology Group Statistics and Data Center), the National Clinical Trials Network Operations Center, and St. Baldrick’s Foundation. See the paper for a complete list of authors and author disclosures.
More recent stories on neuroblastoma
Related Posts :
-

Exposing a tumor’s antigens to enhance immunotherapy
Successful immunotherapy for cancer involves activating a person’s own T cells to attack the tumor. But some tumors have ...
-

Combining CAR-T cells and inhibitor drugs for high-risk neuroblastoma
Chimeric antigen receptor (CAR)-T cell therapy is a potent emerging weapon against cancer, altering patients’ T cells so they ...
-

Could a GI bug’s toxin curb hard-to-treat breast cancer?
Clostridium difficile can cause devastating inflammatory gastrointestinal infections, with much of the damage inflicted by a toxin the bug produces. ...
-

Making immunotherapy safe for AML
Acute myeloid leukemia (AML), the second most common leukemia in children, is hard to treat and has a five-year survival ...