Fetal treatment for vein of Galen malformations
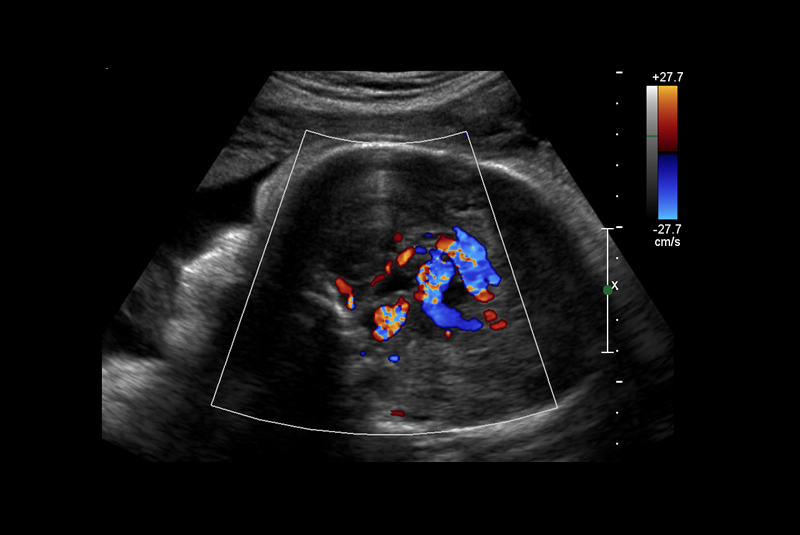
Vein of Galen malformations (VOGMs) are rare, life-threatening vascular malformations that often cause heart failure in neonates. The preferred postnatal treatment for VOGM is transarterial embolization [1]. Although often diagnosed in utero, currently no in utero treatments exist. The Institutional Review Boards (IRBs) at Boston Children’s Hospital and Brigham & Women’s Hospital have agreed that in utero treatment of VOGM may be offered to a small number of patients on a compassionate care basis, in whom there is a predictably grim prognosis, using a current standard of care approach.
What is the prognosis for VOGM?
VOGM present with a broad range of severity, from fulminant cardiopulmonary failure soon after birth to asymptomatic presentation for the first few months of life. Rarely, patients with VOGM may reach adulthood without symptoms.
It is nearly impossible to predict the severity of the postnatal presentation based on prenatal imaging. In our experience, neither the size of the varix nor the number of arterial feeders nor a benign fetal echocardiogram is predictive of benign symptomaticity. Conversely, the presence of parenchymal brain injuries or severe heart failure in utero do predict a nearly uniformly bleak prognosis.
Although great strides have been made with the development of endovascular embolization for the treatment of VOGM, nearly all published outcome studies reflect a highly selected group of patients: those the investigators chose to treat, with the expectation of a potentially positive result. Considering all neonatally diagnosed cases of VOGM, UK investigators found*:
- approximately one-third of all patients do not survive the neonatal period
- approximately one-third suffer moderate to severe neurocognitive compromise despite expert treatment
- only one-third survive to adulthood without significant compromise [2]
*Among published studies from neurovascular specialty treating centers, the UK data are unique in reflecting outcomes across an entire population, with no referral bias. All cases are treated at a designated national center of excellence.
Example case
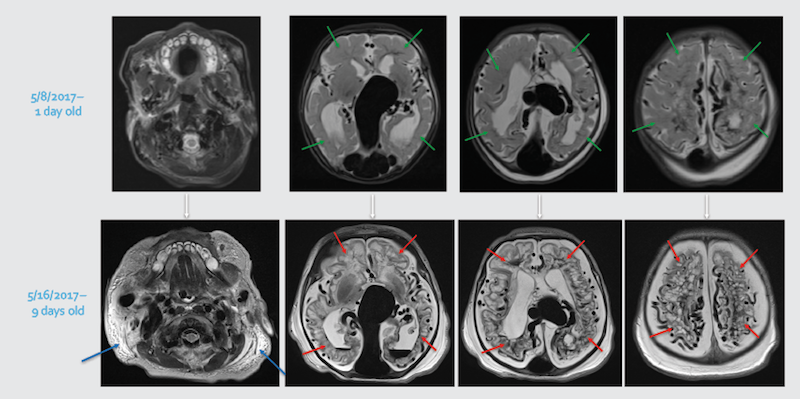
After med-flighting a newborn from Miami to Boston for urgent treatment, MRI obtained at Boston Children’s showed diffuse bihemispheric brain injuries (red arrows), new since the initial MRI (green arrows showing normal brain tissue).
Whole-body edema (anasarca) on the MRI (blue arrows) is secondary to overwhelming heart failure.
Likely no post-natal intervention, even on day one, would have circumvented this rapid evolution.
Why is the prognosis so poor in many cases?
Fetal physiology, with its low-resistance placental circulation, is likely protective of the brain in patients with VOGM:
1. After birth, the low-resistance VOGM collects as much as 70 percent of the cardiac output [3].
2. There is a massive increase in cardiac preload, and with closure of the ductus, pulmonary pressures increase dramatically.
3. The right ventricle dilates and its function becomes impaired. Interventricular septal displacement contributes to left ventricular dysfunction.
4. Diastolic flow reversal is seen in the aorta, due to steal by the VOGM.
5. Cardiac metabolic demands are significantly increased, but coronary artery flow is decreased, resulting in myocardial ischemia and failure [4].
6. Arterial steal from the cerebral circulation is common, likely exacerbated by intracranial venous hypertension, and bihemispheric parenchymal brain injuries begin to accrue.
Why fetal treatment of VOGM?
Given the high rate of treatment failure once the above physiology has taken hold, it is speculated that irreversible changes in the pulmonary vasculature occur early, so that even definitive closure of the VOGM after that point may not ameliorate the pulmonary hypertension.
In all newborns, there is a physiologic steep increase in brain perfusion approximately two days after birth, as an adaptation to postnatal life [5-6]; this is independent of gestational age at delivery [7]. The commonly seen accrual of new parenchymal brain injuries in the first days to weeks postnatally in patients with VOGM and heart failure suggests that this expected uptick in postnatal brain perfusion is lacking in these patients.
Early treatment, before postnatal cardiopulmonary and brain circulatory changes occur, could potentially mitigate the often-fatal pathophysiological cascade, even within this high mortality subgroup.
Embolization of VOGMs by direct coiling of the main venous collector, the median prosencephalic varix, is highly effective, but potentially bears risk in neonates. The fetal environment, where the loss of the low-resistance placental circulation and the significant postnatal increase in overall cerebral circulation have not yet occurred, likely provides a far safer environment for coiling of the prosencephalic varix, allowing rerouting of the deep venous system to occur under conditions of a lower-flow state.
Partial occlusion of the malformation in utero would potentially preempt development of pulmonary hypertension and likely protect both brain and heart from steal-related ischemia. As additional benefits, since no groin arterial access is needed, morbidity to lower-extremity arteries would be avoided, and since the procedure is performed under ultrasound, there is no procedural exposure to ionizing radiation.
The intervention
The procedure is performed in an obstetric operating room , with participation of the maternal-fetal medicine specialist, fetal sonographer, neurointerventionalist, fetal anesthesiologist, and maternal anesthesiologist.
Spinal anesthesia is administered by the maternal anesthesiologist, and the fetus is then positioned for optimal access for treatment. Intramuscular fetal anesthesia is then delivered.
The embolization is performed entirely under ultrasound guidance, accessing the fetal malformation by maternal percutaneous access. The malformation is embolized with detachable platinum coils, of previously calculated numbers and sizes.
The patient is observed overnight with fetal monitoring, and post-procedural fetal echocardiography and fetal MRI are performed.
Postnatal management for this cohort will be identical to current standard of care for VOGM patients: admission to the NICU for close monitoring, along with neonatal echocardiogram and MRI, to ascertain the need for urgent neonatal embolization.
Boston Children’s experience
Boston Children’s interventional cardiology and Brigham & Women’s Hospital maternal-fetal medicine teams have pioneered fetal cardiac interventions, having accrued the largest volume of such procedures worldwide. We have significant expertise in the safe performance of needle-guided fetal interventions, including maternal and fetal safety, fetal anesthesia, and percutaneous needle access to fetal anatomic targets.
Boston Children’s Cerebrovascular Surgery and Interventions Center is a national and international referral center for management of VOGM. We perform 1-2 VOGM embolizations per month in neonates and infants and have the highest volume of pediatric neuroendovascular and open neurosurgical procedures in the U.S. Pediatric neuroanesthesia and neurointensive care function together with the neurointervention and neurosurgery as a combined center of excellence.
The initial cohort: Compassionate care VOGM cases
The IRBs at Brigham & Women’s and Boston Children’s have agreed that in utero treatment of VOGM may be offered to a small number of patients on a compassionate care basis, in whom there is a predictably grim prognosis, using a current standard of care approach.
Inclusion criteria are designed to target specifically fetuses with a grim prognosis. Exclusion criteria are designed to ensure that futile therapy is not delivered, and that unacceptable maternal risk is not present.
Inclusion criteria include fetuses with evidence of:
- multi-organ failure
- hydrops
- pulmonary or pericardial effusions
- fetal echocardiogram showing poor ventricular function
- focal, unilateral small brain parenchymal injury
Exclusion criteria include fetuses with evidence of:
- bihemispheric, diffuse brain parenchymal injuries
- ex vacuo brain ventricular dilatation due to gliosis
- diffuse white matter injury or intracranial hemorrhage
- maternal coagulopathy or other maternal contraindication to fetal therapy
For more updated information, contact Darren Orbach, MD, PhD, co-director of the Cerebrovascular Surgery and Interventions Center and chief of Neurointerventional Radiology at Boston Children’s Hospital.
References
1. Kehrer et al., Development of cerebral blood flow volume in preterm neonates during the first two weeks of life. Pediatric Research 2005, 58:927-302.
2. Rennie et al., Course and outcome of post-neonatal presentations of vein of Galen malformation & Outcomes in neonatal vein of Galen malformation. World Federation of Interventional & Therpeutic Neuroradiology Congress: Budapest, Hungary. October 16th 2017.
3. Deloison et al., Hidden mortality of prenatally diagnosed vein of Galen aneurysmal malformation: retrospective study and review of the literature. Ultrsound Obstet Gynecol 2012, 40:652-658.
4. Paladini et al., Vein of Galen aneurysmal malformation (VOGM) in the fetus: retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound Obstet Gynecol 2017, 50:192-199.
5. Chevret et al., Severe cardiac failure in newborns with VOGM. Intensive Care Medicine 2002, 28:1126-30
6. Wong et al., Hemodynamic disturbances associated with endovascular embolization in newborn infants with vein of Galen malformation. Journal of Perinatology 2006. 26:273-8
7. Patel et al., Systemic hemodynamics in infants with vein of Galen malformation: assessment and basis for therapy. Journal of Perinatology 2007. 27:460-63
Related Posts :
-

Finding comfort and answers for twin-twin transfusion syndrome: Shannon’s story
Shannon’s journey through a challenging pregnancy with TTTS (twin-twin transfusion syndrome) was, as she puts it, an emotional rollercoaster. ...
-

Rowan the Remarkable: Defying the odds with CPAM
This is the story of a baby named Rowan and his remarkable journey of beating the odds after doctors discovered ...
-

A model patient: Alexia’s triumph over moyamoya disease
If you’re lucky enough to get time on Alexia’s packed schedule, you’re in the company of a ...
-

From pain to purpose: An update on Dylan’s traumatic brain injury
We first introduced you to Dylan four years ago, when a traumatic brain injury led him to the Department of ...





